Abstract
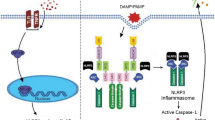
The world’s number one cause of death is cardiovascular diseases. The pathogenesis of different disease entities in the cardiovascular disease spectrum is complicated and multifactorial. Inflammation in these complicated etiologies serves as a key position and is a significant cause of atherosclerosis, which contributes to the underlying pathology. Therefore, therapeutic targeting of inflammatory pathways in patients with cardiovascular diseases such as atherosclerosis enhances cardiovascular results. Inflammasomes are intracellular protein complexes engaged in atherosclerosis pathogenesis and activated by multiple danger signals. Emerging proof has revealed that Nod-like receptor protein 3 (NLRP3) inflammasome, which regulates caspase-1 activation and later pro-interleukin processing, triggers inflammatory reactions in the vascular wall and leads to atherosclerotic plaque formation. Inflammasome-mediated signaling interference could decrease inflammation and mitigate illness severity. In this section, we provide an overview of the present literature on the underlying mechanisms leading to the activation of NLRP3 inflammasome and the role of NLRP3 inflammasome in the progression of atherogenesis and highlight the possibility of therapeutic interventions due to mechanisms involved in the of inhibition of NLRP3 activation.



Similar content being viewed by others
References
Medzhitov, R. 2008. Origin and physiological roles of inflammation. Nature. 454: 428–435.
Lamkanfi, M., and V.M. Dixit. 2014. Mechanisms and functions of inflammasomes. Cell. 157: 1013–1022.
Amin, J., D. Boche, and S. Rakic. 2017. What do we know about the inflammasome in humans? Brain Pathol 27: 192–204.
Schroder, K., R. Zhou, and J. Tschopp. 2010. The NLRP3 inflammasome: A sensor for metabolic danger? Science. 327: 296–300.
Libby. Interleukin-1 beta as a target for atherosclerosis therapy: Jour of the Amer Colle of Cardio. 2017: 18; 2278–2289.
Broz, P., and V.M. Dixit. 2016. Inflammasomes: Mechanism of assembly, regulation and signaling. Nat Rev Immunol 16: 407–420.
Vanaja, S.K., V.A. Rathinam, and K.A. Fitzgerald. 2015. Mechanisms of inflammasome activation: Recent advances and novel insights. Trends Cell Biol 25: 308–315.
Bauernfeind, F.G., et al. 2009. Cutting edge: NF-κB activating pattern recognition and cytokine receptors license NLRP3 inflammasome activation by regulating NLRP3 expression. J Immunol 183: 787–791.
Lemmers, B., L. Salmena, N. Bidère, H. Su, E. Matysiak-Zablocki, K. Murakami, P.S. Ohashi, A. Jurisicova, M. Lenardo, R. Hakem, and A. Hakem. 2007. Essential role for caspase-8 in toll-like receptors and NFκB signaling. J Biol Chem 282: 7416–7423.
Gurung, P., P.K. Anand, R.K.S. Malireddi, L. Vande Walle, N. van Opdenbosch, C.P. Dillon, R. Weinlich, D.R. Green, M. Lamkanfi, and T.D. Kanneganti. 2014. FADD and caspase-8 mediate priming and activation of the canonical and noncanonical Nlrp3 inflammasomes. J Immunol 192: 1835–1846.
Py, B.F., et al. 2013. Deubiquitination of NLRP3 by BRCC3 critically regulates inflammasomes activity. Mol Cell 49: 331–338.
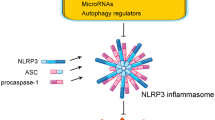
Bauernfeind, F., A. Rieger, F.A. Schildberg, P.A. Knolle, J.L. Schmid-Burgk, and V. Hornung. 2012. NLRP3 inflammasome activity is negatively controlled by miR-223. J Immunol 189: 4175–4181.
Haneklaus, M., J.D. O’Neil, A.R. Clark, S.L. Masters, and L.A.J. O’Neill. 2017. The RNA-binding protein tristetraprolin (TTP) is a critical negative regulator of the NLRP3 inflammasome. J Biol Chem 292: 6869–6881.
Stutz, A., C.C. Kolbe, R. Stahl, G.L. Horvath, B.S. Franklin, O. van Ray, R. Brinkschulte, M. Geyer, F. Meissner, and E. Latz. 2017. NLRP3 inflammasome assembly is regulated by phosphorylation of the pyrin domain. J Exp Med 214: 1725–1736.
Hernandez-Cuellar, E., K. Tsuchiya, H. Hara, R. Fang, S. Sakai, I. Kawamura, S. Akira, and M. Mitsuyama. 2012. Nitric oxide inhibits the NLRP3 inflammasome. J Immunol 189: 5113–5117.
Muñoz-Planillo, R., P. Kuffa, G. Martínez-Colón, B.L. Smith, T.M. Rajendiran, and G. Núñez. 2013. K+ efflux is the common trigger of NLRP3 inflammasome activation by bacterial toxins and particulate matter. Immunity. 38: 1142–1153.
He, Y., M. Zeng, D. Yang, B. Motro, and G. Núñez. 2016. NEK7 is an essential mediator of NLRP3 activation downstream of potassium efflux. Nature. 530: 354–357.
Karmakar, M., M.A. Katsnelson, G.R. Dubyak, and E. Pearlman. 2016. Neutrophil P2X7 receptors mediate NLRP3 inflammasome-dependent IL-1β secretion in response to ATP. Nat Commun 7: 10555.
Katsnelson, M.A., L.G. Rucker, H.M. Russo, and G.R. Dubyak. 2015. K+ efflux agonists induce NLRP3 inflammasome activation independently of Ca2+ signaling. J Immunol 194: 3937–3952.
Murakami, T., J. Ockinger, J. Yu, V. Byles, A. McColl, A.M. Hofer, and T. Horng. 2012. Critical role for calcium mobilization in activation of the NLRP3 inflammasome. Proc Natl Acad Sci 109: 11282–11287.
Csordás, G., and G. Hajnóczky. 1787. SR/ER–mitochondrial local communication: Calcium and ROS. Biochim Biophys Acta Bioenerg 2009: 1352–1362.
Schorn, C., B. Frey, K. Lauber, C. Janko, M. Strysio, H. Keppeler, U.S. Gaipl, R.E. Voll, E. Springer, L.E. Munoz, G. Schett, and M. Herrmann. 2011. Sodium overload and water influx activate the NALP3 inflammasome. J Biol Chem 286: 35–41.
Verhoef, P.A., S.B. Kertesy, K. Lundberg, J.M. Kahlenberg, and G.R. Dubyak. 2005. Inhibitory effects of chloride on the activation of caspase-1, IL-1β secretion, and cytolysis by the P2X7 receptor. J Immunol 175: 7623–7634.
Compan, V., A. Baroja-Mazo, G. López-Castejón, A.I. Gomez, C.M. Martínez, D. Angosto, M.T. Montero, A.S. Herranz, E. Bazán, D. Reimers, V. Mulero, and P. Pelegrín. 2012. Cell volume regulation modulates NLRP3 inflammasome activation. Immunity. 37: 487–500.
Tang, T., X. Lang, C. Xu, X. Wang, T. Gong, Y. Yang, J. Cui, L. Bai, J. Wang, W. Jiang, and R. Zhou. 2017. CLICs-dependent chloride efflux is an essential and proximal upstream event for NLRP3 inflammasome activation. Nat Commun 8: 202.
Shimada, K., T.R. Crother, J. Karlin, J. Dagvadorj, N. Chiba, S. Chen, V.K. Ramanujan, A.J. Wolf, L. Vergnes, D.M. Ojcius, A. Rentsendorj, M. Vargas, C. Guerrero, Y. Wang, K.A. Fitzgerald, D.M. Underhill, T. Town, and M. Arditi. 2012. Oxidized mitochondrial DNA activates the NLRP3 inflammasome during apoptosis. Immunity. 36: 401–414.
Shi, H., Y. Wang, X. Li, X. Zhan, M. Tang, M. Fina, L. Su, D. Pratt, C.H. Bu, S. Hildebrand, S. Lyon, L. Scott, J. Quan, Q. Sun, J. Russell, S. Arnett, P. Jurek, D. Chen, V.V. Kravchenko, J.C. Mathison, E.M.Y. Moresco, N.L. Monson, R.J. Ulevitch, and B. Beutler. 2016. NLRP3 activation and mitosis are mutually exclusive events coordinated by NEK7, a new inflammasome component. Nat Immunol 17: 250–258.
Groß, C.J., R. Mishra, K.S. Schneider, G. Médard, J. Wettmarshausen, D.C. Dittlein, H. Shi, O. Gorka, P.A. Koenig, S. Fromm, G. Magnani, T. Ćiković, L. Hartjes, J. Smollich, A.A.B. Robertson, M.A. Cooper, M. Schmidt-Supprian, M. Schuster, K. Schroder, P. Broz, C. Traidl-Hoffmann, B. Beutler, B. Kuster, J. Ruland, S. Schneider, F. Perocchi, and O. Groß. 2016. K+efflux-independent NLRP3 inflammasome activation by small molecules targeting mitochondria. Immunity. 45: 761–773.
Halle, A., V. Hornung, G.C. Petzold, C.R. Stewart, B.G. Monks, T. Reinheckel, K.A. Fitzgerald, E. Latz, K.J. Moore, and D.T. Golenbock. 2008. The NALP3 inflammasome is involved in the innate immune response to amyloid-β. Nat Immunol 9: 857–865.
Hornung, V., F. Bauernfeind, A. Halle, E.O. Samstad, H. Kono, K.L. Rock, K.A. Fitzgerald, and E. Latz. 2008. Silica crystals and aluminum salts activate the NALP3 inflammasome through phagosomal destabilization. Nat Immunol 9: 847–856.
Wang, Z., W. Hu, C. Lu, Z. Ma, S. Jiang, C. Gu, D. Acuña-Castroviejo, and Y. Yang. 2018. Targeting NLRP3 (nucleotide-binding domain, Leucine-rich–containing family, pyrin domain–Containing-3) inflammasome in cardiovascular disorders. Arterioscler Thromb Vasc Biol 38: 2765–2779.
Karasawa, T., and M. Takahashi. 2017. Role of NLRP3 inflammasomes in atherosclerosis. Review. J Atheroscler Thromb 24: 000–000.
Zheng, F., S. Xing, Z. Gong, and Q. Xing. 2013. NLRP3 inflammasomes show high expression in aorta of patients with atherosclerosis. Heart Lung Circ 22: 746–750.
Varghese, G.P., L. Folkersen, R.J. Strawbridge, et al. 2016. NLRP3 inflammasome expression and activation in human atherosclerosis. J Am Heart Assoc 5: 3031.
Shi, X., W.L. Xie, W.W. Kong, D. Chen, and P. Qu. 2015. Expression of the NLRP3 inflammasome in carotid atherosclerosis. J Stroke Cerebrovasc Dis 24: 2455–2466.
Duewell, P., H. Kono, K.J. Rayner, C.M. Sirois, G. Vladimer, F.G. Bauernfeind, G.S. Abela, L. Franchi, G. Nuñez, M. Schnurr, T. Espevik, E. Lien, K.A. Fitzgerald, K.L. Rock, K.J. Moore, S.D. Wright, V. Hornung, and E. Latz. 2010. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature. 464: 1357–1361.
Hendrikx, T., M.L.J. Jeurissen, P.J. Van Gorp, et al. 2015. Bone marrow-specific caspase-1/11 deficiency inhibits atherosclerosis development in Ldlr−/− mice. FEBS J 282: 2327–2338.
Wang, R., Y. Wang, and N. Muetal. 2017. Activation of NLRP3inflammasomes contributes to hyperhomocysteinemia-aggravated inflammation and atherosclerosis in apoE-deficient mice. Lab Investig 97: 922–934.
Ding, Z., S. Liu, X. Wang, Y. Dai, M. Khaidakov, X. Deng, Y. Fan, D. Xiang, and J.L. Mehta. 2014. LOX-1, mtDNA damage, and NLRP3 inflammasome activation in macrophages: Implications in atherogenesis. Cardiovasc Res 103: 619–628.
Mehta, J.L., N. Sanada, C.P. Hu, et al. 2007. Deletion of LOX-1 reduces atherogenesis in LDLR knockout mice fed high cholesterol diet. J Circ Res 100: 1634–1642.
Chen, L., Q. Yao, S. Xu, H. Wang, and P. Qu. 2018. Inhibition of the NLRP3 inflammasome attenuates foam cell formation of THP-1 macrophages by suppressing ox-LDL uptake and promoting cholesterol efflux. Biochem Biophys Res Commun 495: 382–387.
Tumurkhuu, G., K. Shimada, J. Dagvadorj, et al. 2016. Ogg1-dependent DNA repair regulates NLRP3 inflammasome and prevents atherosclerosis. Circ Res 119: 76–90.
Wang, Y., Z. Han, Y. Fan, J. Zhang, K. Chen, L. Gao, H. Zeng, J. Cao, and C. Wang. 2017. MicroRNA-9 inhibits NLRP3 inflammasome activation in human atherosclerosis inflammation cell models through the JAK1/STAT signaling pathway. Cell Physiol Biochem 41: 1555–1571.
Rajamaki, K., J. Lappalainen, K. Oorni, et al. 2010. Cholesterol crystals activate the NLRP3 inflammasome in human macrophages: a novel link between cholesterol metabolism and inflammation. PLoS One 7: e11765.
Tabas, I., and A.H. Lichtman. 2017. Monocyte-macrophages and T cells in atherosclerosis. Immun. 4: 621–634.
Hendrikx T, Jeurissen M.L.J, van Gorp P. J, et al. Bone marrow-specific caspase-1/11 deficiency inhibits atherosclerosis development inLdlr−/− mice. FEBS J. 2015;12:2327–2338.
Yin, Y., X. Li, X. Sha, et al. 2015. Early hyperlipidemia promotes endothelial activation via a caspase-1-sirtuin 1 pathway. Arterio, Thromb, and Vascu Bio 35: 804–816.
Wu, X., H. Zhang, W. Qi, et al. 2018. Nicotine promotes atherosclerosis via ROS-NLRP3-mediated endothelial cell pyroptosis. Cell Death & Disease 2: 171.
Mestas, J., and K. Ley. 2008. Monocyte-endothelial cell interactions in the development of atherosclerosis. Trends Cardiovasc Med 6: 228–232.
Sheikine, Y., and A. Sirsjö. 2008. CXCL16/SR-PSOX-A friend or a foe in atherosclerosis? Atherosc 197: 487–495.
Kawaguchi, M., M. Takahashi, T. Hata, Y. Kashima, F. Usui, H. Morimoto, A. Izawa, Y. Takahashi, J. Masumoto, J. Koyama, M. Hongo, T. Noda, J. Nakayama, J. Sagara, S.’. Taniguchi, and U. Ikeda. 2011. Inflammasome activation of cardiac fibroblasts is essential for myocardial ischemia/reperfusion injury. Cir. 123: 594–604.
Fang, L., K.K. Wang, P.F. Zhang, T. Li, Z.L. Xiao, M. Yang, and Z.X. Yu. 2020. Nucleolin promotes Ang II-induced phenotypic transformation of vascular smooth muscle cells by regulating EGF and PDGF-BB. J Cell Mol Med 24: 1917–1933.
Bennett, M.R., S. Sinha, and G.K. Owens. 2016. Vascular smooth muscle cells in atherosclerosis. Circ Res 118: 692–702.
Wen, C., X. Yang, Z. Yan, et al. 2013. Nlrp3 inflammasome is activated and required for vascular smooth muscle cell calcification. Int J Cardiol 168: 2242–2247.
Man, S.M., Q. Zhu, L. Zhu, Z. Liu, R. Karki, A. Malik, D. Sharma, L. Li, R.K.S. Malireddi, P. Gurung, G. Neale, S.R. Olsen, R.A. Carter, D.J. McGoldrick, G. Wu, D. Finkelstein, P. Vogel, R.J. Gilbertson, and T.D. Kanneganti. 2015. Critical role for the DNA sensor AIM2 in stem cell proliferation and cancer. Cell. 162: 45–58.
Luchetti, F., R. Crinelli, E. Cesarini, B. Canonico, L. Guidi, C. Zerbinati, G. di Sario, L. Zamai, M. Magnani, S. Papa, and L. Iuliano. 2017. Endothelial cells, endoplasmic reticulum stress and oxysterols. Redox Biol 13: 581–587.
Çimen, I., B. Kocatürk, S. Koyuncu, et al. 2016. Prevention of atherosclerosis by bioactive palmitoleate through suppression of organelle stress and inflammasome activation. Sci Transl Med 8: 126.
Rsazani, B., C. Feng, T. Coleman, et al. 2012. Autophagy links inflammasomes to athero- sclerotic progression. Cell Metab 15: 534–544.
Pols, T.W., M. Nomura, T. Harach, et al. 2011. TGR5 activation inhibits atherosclerosis by reducing macrophage inflammation and lipid loading. Cell Metab 14: 747–757.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• Cardiovascular diseases involving heart and vasculature disorders are a serious global health burden that is presently the world’s leading cause of death.
• Multiple danger signals such as cholesterol crystals, calcium phosphate crystals, and oxidized low-density lipoprotein triggered NLRP3 inflammasome activation in macrophages to initiate inflammatory reactions in atherosclerotic lesions.
• Understanding NLRP3 inflammasome activation mechanisms will allow its particular inhibitors to be developed to treat NLRP3-related illnesses
Electronic supplementary material
ESM 1
(DOCX 96.9 kb)
Rights and permissions
About this article
Cite this article
Liaqat, A., Asad, M., Shoukat, F. et al. A Spotlight on the Underlying Activation Mechanisms of the NLRP3 Inflammasome and its Role in Atherosclerosis: A Review. Inflammation 43, 2011–2020 (2020). https://doi.org/10.1007/s10753-020-01290-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-020-01290-1