- 1Clinical and Translational Epidemiology Unit, Mongan Institute, Massachusetts General Hospital, Boston, MA, United States
- 2Department of Medicine, Harvard Medical School, Boston, MA, United States
- 3Programs in Metabolism and Medical & Population Genetics, Broad Institute of MIT and Harvard, Cambridge, MA, United States
- 4Center for Molecular Medicine & Genetics, Wayne State University, Detroit, MI, United States
- 5Karmanos Cancer Institute at Wayne State University, Detroit, MI, United States
- 6Center for Genomic Medicine, Massachusetts General Hospital, Boston, MA, United States
- 7Division of General Internal Medicine, Massachusetts General Hospital, Boston, MA, United States
- 8Diabetes Unit, Massachusetts General Hospital, Boston, MA, United States
- 9Department of Statistics, University of California, Berkeley, Berkeley, CA, United States
- 10Centre for Computational Biology, University of Birmingham, Birmingham, United Kingdom
- 11Computational Biosciences Group, Biosciences Area, Lawrence Berkeley National Laboratory, Berkeley, CA, United States
- 12Division of Endocrinology, Diabetes, and Metabolism, Department of Medicine, Cedars-Sinai Medical Center, Los Angeles, CA, United States
- 13The Institute for Translational Genomics and Population Sciences, Department of Pediatrics, The Lundquist Institute for Biomedical Innovation at Harbor-UCLA Medical Center, Torrance, CA, United States
- 14Department of Human Genetics, McGill University, Montréal, QC, Canada
- 15Department of Medicine, McGill University, Montréal, QC, Canada
- 16McGill University and Genome Québec Innovation Centre, Montréal, QC, Canada
- 17Department of Neurology, School of Medicine, Wayne State University, Detroit, MI, United States
Aims: Causal transcripts at genomic loci associated with type 2 diabetes (T2D) are mostly unknown. The chr8p23.1 variant rs4841132, associated with an insulin-resistant diabetes risk phenotype, lies in the second exon of a long non-coding RNA (lncRNA) gene, LOC157273, located 175 kilobases from PPP1R3B, which encodes a key protein regulating insulin-mediated hepatic glycogen storage in humans. We hypothesized that LOC157273 regulates expression of PPP1R3B in human hepatocytes.
Methods: We tested our hypothesis using Stellaris fluorescent in situ hybridization to assess subcellular localization of LOC157273; small interfering RNA (siRNA) knockdown of LOC157273, followed by RT-PCR to quantify LOC157273 and PPP1R3B expression; RNA-seq to quantify the whole-transcriptome gene expression response to LOC157273 knockdown; and an insulin-stimulated assay to measure hepatocyte glycogen deposition before and after knockdown.
Results: We found that siRNA knockdown decreased LOC157273 transcript levels by approximately 80%, increased PPP1R3B mRNA levels by 1.7-fold, and increased glycogen deposition by >50% in primary human hepatocytes. An A/G heterozygous carrier (vs. three G/G carriers) had reduced LOC157273 abundance due to reduced transcription of the A allele and increased PPP1R3B expression and glycogen deposition.
Conclusion: We show that the lncRNA LOC157273 is a negative regulator of PPP1R3B expression and glycogen deposition in human hepatocytes and a causal transcript at an insulin-resistant T2D risk locus.
Introduction
Type 2 diabetes (T2D), a continually growing scourge worldwide, arises from the interaction of multiple factors with genetic susceptibility in insulin sensitivity and secretion pathways to increase risk (Knowler et al., 1981; Narayan et al., 2006; Cauchi et al., 2008). The search for genetic determinants of T2D and its risk factors has revealed over 400 common variants at over 250 coding and regulatory genomic loci that influence multiple distinct aspects of T2D pathophysiology (Manning et al., 2012; Wessel et al., 2015; Fuchsberger et al., 2016; Mahajan et al., 2018a, b).
In quantitative trait genome-wide association studies (GWAS) of non-diabetic individuals, we showed that the minor (rs4841132-A) allele at the chromosome 8p23.1 variant rs4841132 (NR_040039.1:n.548A > G; reference allele A has frequency ∼11%) was significantly associated with an insulin resistance phenotype characterized by increased levels of fasting glucose (FG) and insulin (FI), elevated levels of triglycerides, and an increased waist–hip ratio (Manning et al., 2012). This chromosome 8 locus is highly pleiotropic, and rs4841132 and nearby SNPs have been consistently associated with increased T2D risk as well as T2D-related metabolic phenotypes including glycemia in pregnancy, obesity, HDL:LDL ratio, total cholesterol, triglycerides, C-reactive protein levels, coronary artery disease, subclinical atherosclerosis, and fatty liver disease (Lettre et al., 2011; Speliotes et al., 2011; Inouye et al., 2012; Manning et al., 2012; Hayes et al., 2013; Willer et al., 2013; Ligthart et al., 2015; Raffield et al., 2017).
The variant rs4841132 resides ∼175 kb from the nearest protein-coding gene, PPP1R3B. PPP1R3B encodes the glycogen-targeting subunit of PP1 protein phosphatase and is expressed most strongly in liver in both rodents and man, and at lower levels in skeletal muscle and other tissues (Gasa et al., 2000, 2002; Newgard et al., 2000). PPP1R3B connects ambient insulin to hepatic glycogen regulation: its overexpression in hepatocytes markedly increases both basal and insulin-stimulated glycogen synthesis (Agius, 2015). PPP1R3B has long been an attractive target for diabetes therapy, based on the concept of tipping ambient glycemic balance toward hepatic glycogen deposition (Cohen, 2006; Gasa et al., 2002).
We previously localized rs4841132 to exon 2 of a previously unannotated long non-coding RNA (lncRNA) gene, LOC157273 (ENSG00000254235.1; NR_040039.1) (Liu et al., 2016). In ancestry-specific analyses, we identified a second variant rs9949 (chr8 distance 189084 kb; r2YRI with rs4841132, 0.18; r2CEU, 0.01) that resided in the second exon of PPP1R3B and was weakly associated with FI (P = 6.9 × 10–5) (Liu et al., 2016) and T2D (P = 5.9 × 10–4) in African ancestry individuals. The lncRNA encoded at LOC157273 is a plausible effector transcript for both variants, as lncRNAs are highly enriched at trait- and disease-associated loci, a few are now known to regulate metabolic pathways and disease risk, and many lncRNAs are cis-regulators, exerting both positive and negative regulation of neighboring protein-coding genes (Katayama et al., 2005; Engstrom et al., 2006; Ishii et al., 2006; Orom et al., 2010; Moran et al., 2012; Mitchel et al., 2016; Tajbakhsh et al., 2016).
These observations support a potential genetic regulatory relationship between LOC157273 and PPP1R3B that could explain the observed metabolic trait and T2D risk GWAS associations at the chr8p23.1 locus (Dongiovanni et al., 2018; Stender et al., 2018). As PPP1R3B is abundantly expressed in the human liver where it regulates glycogen storage, we studied cultured human hepatocytes to test the hypothesis that LOC157273 regulates PPP1R3B expression, and consequently insulin-mediated glycogen deposition, and that LOC157273 regulation of PPP1R3B varies by genotype at rs4841132.
Materials and Methods
SNP Genotyping
We obtained primary human hepatocytes from commercial sources and genotyped them by sequencing the region surrounding rs4841132 in a 2.9-kb LOC157273 amplicon from purified DNA (Supplementary Text). We identified one rs4841132 A/G heterozygote out of 16 available hepatocyte donors (Supplementary Table S1 and Figure S1). This study was not considered human subjects research, as the research was performed using de-identified biospecimens from deceased individuals that were commercially obtained from Lonza (formerly Triangle Research Labs) or Thermo Fisher Scientific (formerly LifeTech).
Cellular Localization of LOC157273 With Stellaris RNA Fluorescent in situ Hybridization
We used a custom-synthesized 48-probe set (LGC Biosearch Technologies; Petaluma, CA) of non-overlapping fluorescent-tagged oligonucleotides that tiled the 3.4-kb LOC157273 transcript. Probe nucleotide choices at all polymorphic sites were based on the NR_040039.1 reference transcript, which contains the rs4841132-A (minor) allele. Fixed cells grown on collagen-coated glass coverslips were probed with the pooled probe set following the Biosearch Technologies Stellaris fluorescent in situ hybridization (FISH) protocol for adherent cells.1 After mounting using Vectashield with DAPI, the hybridized coverslips were examined under an AxioObserver inverted fluorescence microscope (Carl Zeiss Microscopy) equipped with a 63 × /1.40 oil objective lens. Red bodies in the merged images denote the Quasar 570 signal from the LOC157273 molecules; the blue-colorized DAPI staining shows cell nuclei. Greater detail is provided in the Supplementary Material.
TaqMan Quantitative Reverse-Transcriptase PCR to Measure the Expression Levels of Two Isoforms of PPP1R3B mRNA and a Single Isoform of LOC157273 lncRNA
Oligo(dT) priming was used for reverse transcription of RNA into cDNA for all Taqman quantitative reverse-transcriptase PCR (qRT-PCR) analyses. Primers and probe sets are described in more detail in the Supplementary Text and Table S2. The LOC157273 amplicon spans intron 1 and includes 36 nt of exon 1 and 41 nt of exon 2. PPP1R3B transcription was assessed with two probe sets—one for the hepatocyte-specific mRNA isoform, and one for the (more ubiquitous) mRNA isoform (Supplementary Table S3). Three or four biological replicates were obtained for Taqman qRT-PCR in primary human hepatocyte donors TRL4079, Hu8200, TRL4056B, TRL4105A, and TRL4108. All reactions were run as technical triplicates using the Applied Biosystems 7500 Fast Real-Time Instrument.
Small Interfering RNA Knockdown of LOC157273
Small interfering RNA (siRNA) knockdown of LOC157273 was performed on primary human hepatocytes from donor Hu8200 (genotype G/G at rs4841132) plated into collagen-coated 6-well plates (Thermo Fisher Scientific #A11428-01). Cells were incubated in complete Williams’ E medium and transfected with siRNA (50 nM; Dharmacon) and Lipofectamine® RNAiMAX reagent (Thermo Fisher Scientific #13778075) in a final concentration of 1 ml/well Opti-MEMTM. The target sequences in the 3.4-kb LOC157273 lncRNA (NR_040039.1 or Ensembl ENST00000520390.1) were as follows: siRNA09 in the 3′-end of the third exon (GGGAAGGGTTAGAGAGGTC), siRNA11 in the first exon (CAACTTAGCTTCTCCATTTTT), siRNA13 near the 5′-end of the third exon (AGAGAAGGACTGAAGATCATT), and siRNA15 in the second exon (TCAGAGGACTTGACACCAT) where the sequences represent the sense-strand DNA targeted by the siRNAs. Six hours after transfection, 1 ml of complete medium (including 1X HepExtend) was pipetted into each well. On the next day, medium was removed after gentle up-and-down pipetting (trituration) to dislodge dead cells, and the transfected monolayer was supplemented first with 1 ml of complete Williams’ E and then with ice-cold complete medium (including HepExtend) containing 1 mg/ml fibronectin (Thermo Fisher Scientific Geltrex #A1413202), followed by gentle trituration to mix the fibronectin (final concentration becomes 0.5 mg/ml). Complete medium with HepExtend was changed daily in the evening (with gentle trituration) until 120 h after initial plating. We performed pooled analysis of TaqMan qRT-PCR results from three biological replicates of the siRNA knockdown experiment after applying sequential corrections, including log transformation, mean centering, and autoscaling (Dechamethakun and Muramatsu, 2017).
Transcriptome-Wide Effects of LOC157273 Knockdown Using RNA Sequencing
Transcriptome sequencing was performed by the Broad Institute’s Sequencing Platform (Ardlie et al., 2015). Using the NCBI refGene database (hg19) and R/Bioconductor packages (GenomicFeatures, rsubread), we collapsed transcript annotations into genes and obtained gene counts using the pairedEnd option in featureCounts for all experimental treatment groups. Three biological replicates were performed using primary human hepatocytes (Hu8200 donor) for four different siRNAs (siRNA09, siRNA11, siRNA13, and siRNA15) and for control experiments consisting of either mock or scrambled siRNA transfection. We examined batch effects during QC of the RNA-seq data and observed that the samples clustered by batch in a principle component analysis (PCA) analysis of the “regularized log transformed” gene counts. We used a variance stabilizing transformation of the gene counts and applied the “removeBatchEffect” function from the limma package. PCA analysis of the batch-normalized gene counts subsequently showed clusters defined by experiment label (mock or scramble; siRNA11 or siRNA15). Differentially expressed genes were obtained with DESeq2, with adjustment for batch effects (due to the observations during sample QC) in a model comparing gene counts in siRNA11 and siRNA15 experimental conditions to mock and scramble controls. We observed low counts, which is standard when analyzing transcriptome-wide expression of protein-coding genes and lncRNAs even in the absence of siRNA knockdown. Therefore, we utilized shrinkage based on re-estimating the variance using dispersion estimates with a negative binomial distribution (Anders and Huber, 2010; McCarthy et al., 2012; Wu et al., 2013). In order to understand similarities among genes with relatively large effects, we created a subset of genes with DESeq2 adjusted P < 0.001 and fold changes greater than the observed fold change for PPP1R3B. We performed hierarchical clustering with batch-normalized gene counts and Reactome pathway-based analysis with this gene subset. We also performed gene-set enrichment analysis using all genes with the ReactomePA R package (Yu and He, 2016).
Glycogen Deposition Assay in Response to Insulin or Glucagon
We developed a protocol to measure glycogen content in cultured primary human hepatocytes using donor TRL4079 (heterozygote A/G at rs4841132) and donors TRL4055A, TRL4113, and TRL4012 (homozygous G/G at rs4841132). In the assay, adapted from Gómez-Lechón et al. (1996), Aspergillus niger amyloglucosidase (Sigma-Aldrich #A7420) degrades cell-derived glycogen to glucose, which was measured in a fluorescent peroxide/peroxidase assay. Cells were lysed with ice-cold solubilization buffer (2% CHAPS, 150 mM NaCl, and 25 mM Tris–HCl, pH 7.2) containing 1 × HALTTM protease inhibitor cocktail (Thermo Fisher Scientific 78430), using 400 μl of lysis buffer for each well of a 6-well plate. To measure glucose polymerized as glycogen, the lysate was diluted 1:10 with sodium acetate buffer (50 mM Na-acetate, pH 5.5) and treated with either 0.75–1.5 U amyloglucosidase (Sigma A7420) at pH 5.5 (60 min at 37°C) or pH 5.5 buffer without enzyme. After incubation, 5-μl aliquots of the ± enzyme reactions were pipetted in triplicate (or more) into black 96-well plates (Corning #3603). Subsequently, 45 μl of a cocktail of glucose oxidase, horseradish peroxidase, and AmplexRed (10-acetyl-3,7-dihydroxyphenoxazine; from the components of Molecular Probes kit A22189) were added to the samples and maintained at room temperature in the dark until reading in the Synergy H1 instrument (Biotek) at 80% gain, fluorescence endpoint, excitation 530 nm, emission 590 nm. The fluorescence values from the negative controls were subtracted from experiment values to estimate the amount of glucose released from glycogen by amyloglucosidase.
Optimal establishment of primary human hepatocytes in tissue culture required a substratum of type 1 collagen (24-well plates; Thermo Fisher Scientific #A11428-02) and initial plating at 200,000 cells/well in complete Williams’ E medium containing supraphysiological concentrations of insulin. This medium was essential for high-efficiency plating but precluded the study of insulin effect. We developed an insulin-free DMEM (IF-DMEM) supplemented with nicotinamide, zinc, copper, glutamine, transferrin, selenous acid, and dexamethasone and no serum (Block et al., 1996), which replaced the complete Williams’ E from Day 2 onward (after washout of insulin from the adherent monolayers). After 24 h in IF-DMEM, we re-stimulated monolayers with 5000 pM insulin (fast-acting lispro insulin; Humalog from Lilly) for 24 h and cellular glycogen content was assessed. Glucagon (GCG) treatment was for 15 or 30 min. Glucagon-mediated glycogenolysis was complete by 15 min with no change thereafter. Each glycogen assay included a glucose standard curve (0–5 nmol) as an absolute reference against which we gauged the fluorescence from Resorufin in insulin or GCG-treated primary hepatocytes and used to estimate glycogen content in nanomolar. We also assessed the effect of siRNA knockdown of LOC157273 on glycogen storage in hepatocytes from donor Hu8200 using the same protocol. A generalized linear model was used to estimate the mean effect of siRNA11 and siRNA15 on hepatocyte glycogen content in nanomolar.
Allelic Imbalance of LOC157273 Transcription in Primary Human Hepatocytes
LOC157273 allelic imbalance was measured using RNA from primary human hepatocytes from a heterozygous (A/G) donor. We estimated allele-specific LOC157273 transcription using gene-specific strand-specific (GSSS) reverse-transcription followed by PCR (Supplementary Text) and analysis on ethidium bromide stained (EtBr-stained) agarose gels. cDNA priming was performed with a gene-specific primer (Supplementary Table S3) targeting a region of exon 3 common to major and minor alleles. The discrimination between major (rs4841132-G) and minor (rs4841132-A) alleles relies on the subsequent PCR step utilizing the reverse primers (32A, 32G, 33A, or 33G) where the most 3′-base in the PCR primer is T (for 32A or 33A) or C (for 32G or 33G)—the precise position of the SNP.
Results
Bioinformatic Evidence That LOC157273 Is a Candidate Causal Transcript
Bioinformatic analysis showed the chr8p23.1 PPP1R3B-LOC157273 locus to have the greatest amount of GWAS evidence for disease associations after intersection with all lncRNA genes (Supplementary Text and Table S4). The variant rs4841132 lies in a linkage disequilibrium (LD) region that spans LOC157273 (Supplementary Figure S2) at a location corresponding to a promoter-like histone state in liver-derived cells (Supplementary Table S5 and Text). The strongest DNase I hypersensitive site lies at the conserved promoter of LOC157273 (the gene appears to be conserved only between humans and non-human primates) and contains CEBP and FOXA1 binding sites (Supplementary Figure S3). LOC157273 is expressed almost exclusively in human hepatocytes (Supplementary Figure S3; Forrest et al., 2014). Notably, human LOC157273 (hg19 and hg38) does not have any positional equivalents or putative orthologs in mouse (mm9 and mm10) detectable using our approaches (Supplementary Figures S4A,B; Wood et al., 2013). Evident lack of conservation beyond primates is typical for human lncRNA genes (Font-Cunill et al., 2018; Levin, 2019), and primate specificity of functional lncRNAs such as LOC157273 hints at limitations of mouse models.
siRNA Knockdown of LOC157273 Reduces LOC157273 and Increases PPP1R3B RNA Levels and Glycogen Deposition in Human Hepatocytes
Stellaris RNA FISH in rs4841132 G/G or A/G human hepatocytes showed that LOC157273 is a cytoplasmic lncRNA, confined to small punctate (0.5–1.2 μm) bodies surrounding the nuclei (Figure 1). As cytoplasmic lncRNAs are amenable to siRNA-mediated knockdown, in G/G hepatocytes, we performed transient transfection of four different siRNAs targeting exons 1, 2, and 3 of the LOC157273 transcript (Figure 2A). The siRNAs siRNA-09 and siRNA-13 did not reproducibly reduce LOC157273 transcript levels, but siRNA-11 and siRNA-15 reproducibly decreased LOC157273 lncRNA by 72% [95% confidence interval (CI): 70–74%] and 75% (95% CI: 73–76%), respectively (Figure 2B). Knockdown with siRNA-11 and siRNA-15 increased the level of PPP1R3B mRNA by 57% (95% CI: 54–61%) and 79% (95% CI: 70–89%), respectively (Figure 2C).
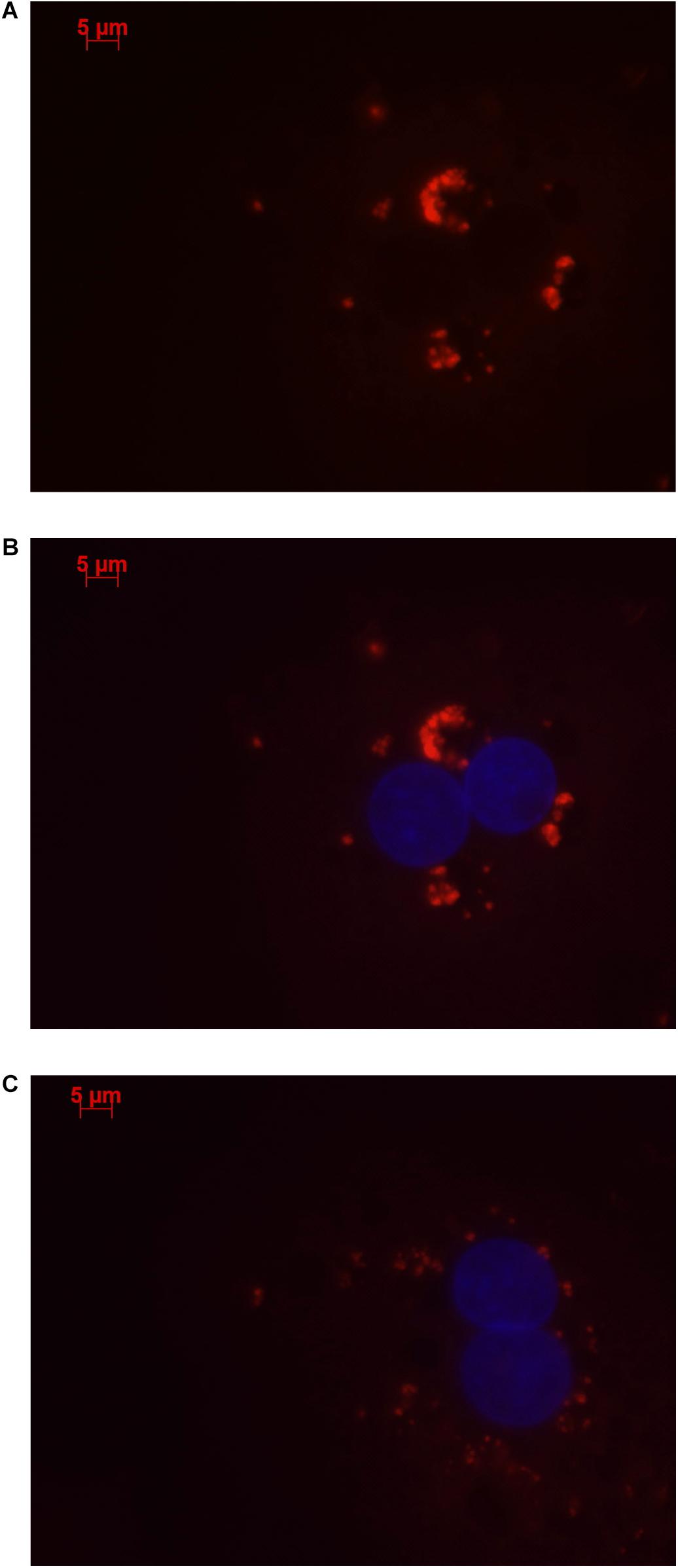
Figure 1. Human hepatocytes stained using Stellaris RNA FISH show that lncRNA LOC157273 localizes to distinct punctate foci in the cytoplasm. The red staining (63 × oil, 500 ms, 568 nm) in human hepatocytes with rs4841132 G/G genotype (A,B) or with rs4841132 A/G genotype (C) shows cytoplasmic punctate foci containing LOC157273, a pattern consistent with the possible localization of this lncRNA in cytoplasmic riboprotein (protein–RNA) complexes. Blue staining indicates hepatocyte nuclei.
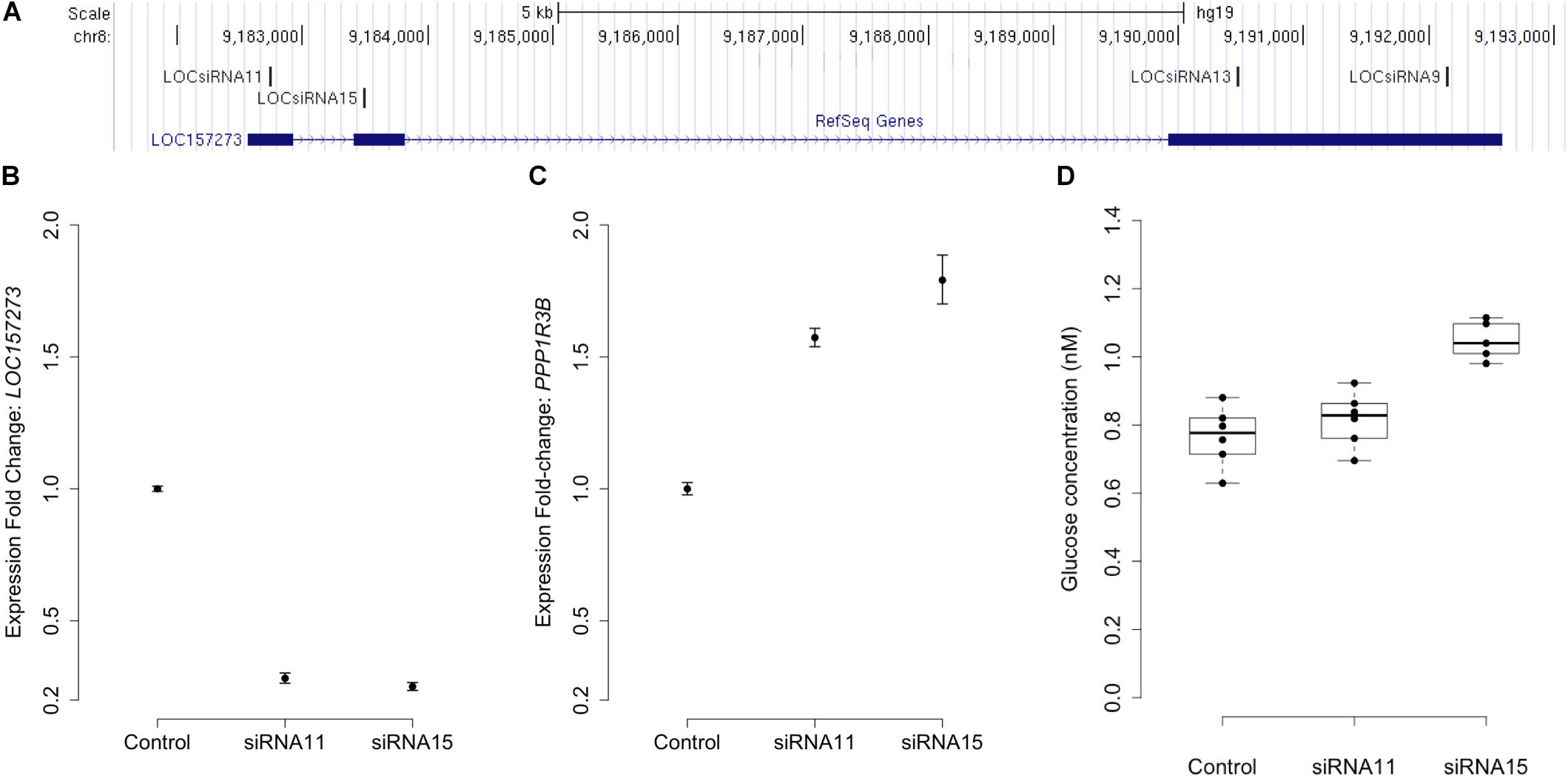
Figure 2. siRNA knockdown of LOC157273 in human hepatocytes with rs4841132 G/G genotype reduces LOC157273 lncRNA levels and increases both PPP1R3B mRNA levels and glycogen deposition. Panel (A) shows a UCSC Genome Browser view of the genomic position on chromosome 8 (hg19) of LOC157273 (sense) and four siRNA constructs (antisense). siRNA-11 and siRNA-15, the most efficient constructs, targeted exons 1 and 2 of the lncRNA. Panel (B) shows expression fold change of LOC157273 mRNA and Panel (C) shows PPP1R3B mRNA, after knockdown with siRNA11, siRNA15, and control. Error bars represent standard errors of normalized Taqman qRT-PCR expression normalized and averaged over three biological replicates. Panel (D) shows human hepatocyte glycogen content (six replicates per condition) after knockdown with siRNA11, siRNA15, and control in one biological replicate.
We investigated the effects of LOC157273 knockdown on cell physiology by measuring glycogen production, which is directly affected by PPP1R3B levels. Averaged across three biological replicates (accounting for biological variability), LOC157273 knockdown with siRNA-15 increased insulin-stimulated glycogen deposition by 13% (P = 0.002) (Figure 2D and Supplementary Figure S5). Notably, siRNA-15 is the siRNA located closest to the variant rs4841132 in exon 2 of LOC157273. We also tested plasmid-based overexpression of LOC157273 in human hepatocytes and hepatoma cells but did not find alteration in levels of PPP1R3B expression (Supplementary Text and Figure S6).
As Expected, LOC157273 Knockdown Has Diverse Transcriptome-Wide Effects
We performed a gene expression differential expression analysis of human hepatocyte whole transcriptomes comparing siRNA-11 and siRNA-15 knockdown to control conditions. Of 15,441 unique genes tested, 953 genes showed nominal evidence for differential expression at P < 0.01 (Figure 3A and Supplementary Table S6). RNA-seq results were consistent with Taqman qRT-PCR results for both LOC157273 (fold change = 0.75, Padj = 0.03) and PPP1R3B (fold change = 1.34, Padj = 0.001). To elucidate biological pathways that might be affected by these expression changes, we performed a Reactome pathway analysis with three sets of genes: the 953 genes with Padj < 0.01 (Supplementary Table S7A), the 206 genes with fold change ≤ 0.74 and Padj < 0.001, and the 222 genes with fold change ≥ 1.34 and Padj < 0.001 (Supplementary Figure S7 and Table S7B). We observed two nominally significant Reactome pathways with the genes with fold change ≤ 0.74 and Padj < 0.001: Glucuronidation and Biological Oxidations (Padj = 0.04 for both pathways), and 17 enriched Reactome pathways with the genes with fold change ≥ 1.34 and Padj < 0.001 (Figure 3B). An unbiased gene set enrichment analysis, using all gene results from the differential expression analysis, showed 164 enriched pathways with Padj < 0.05, including fatty acid metabolism (Padj = 0.0004), glucuronidation (Padj = 0.0007), gluconeogenesis (Padj = 0.02), and glucose metabolism (Padj = 0.03) (Supplementary Table S8). Of the significantly enriched pathways, 23% fall under “Cell Cycle” in the Reactome hierarchy, 16% under “Metabolism” and 12% under “Signal Transduction.”
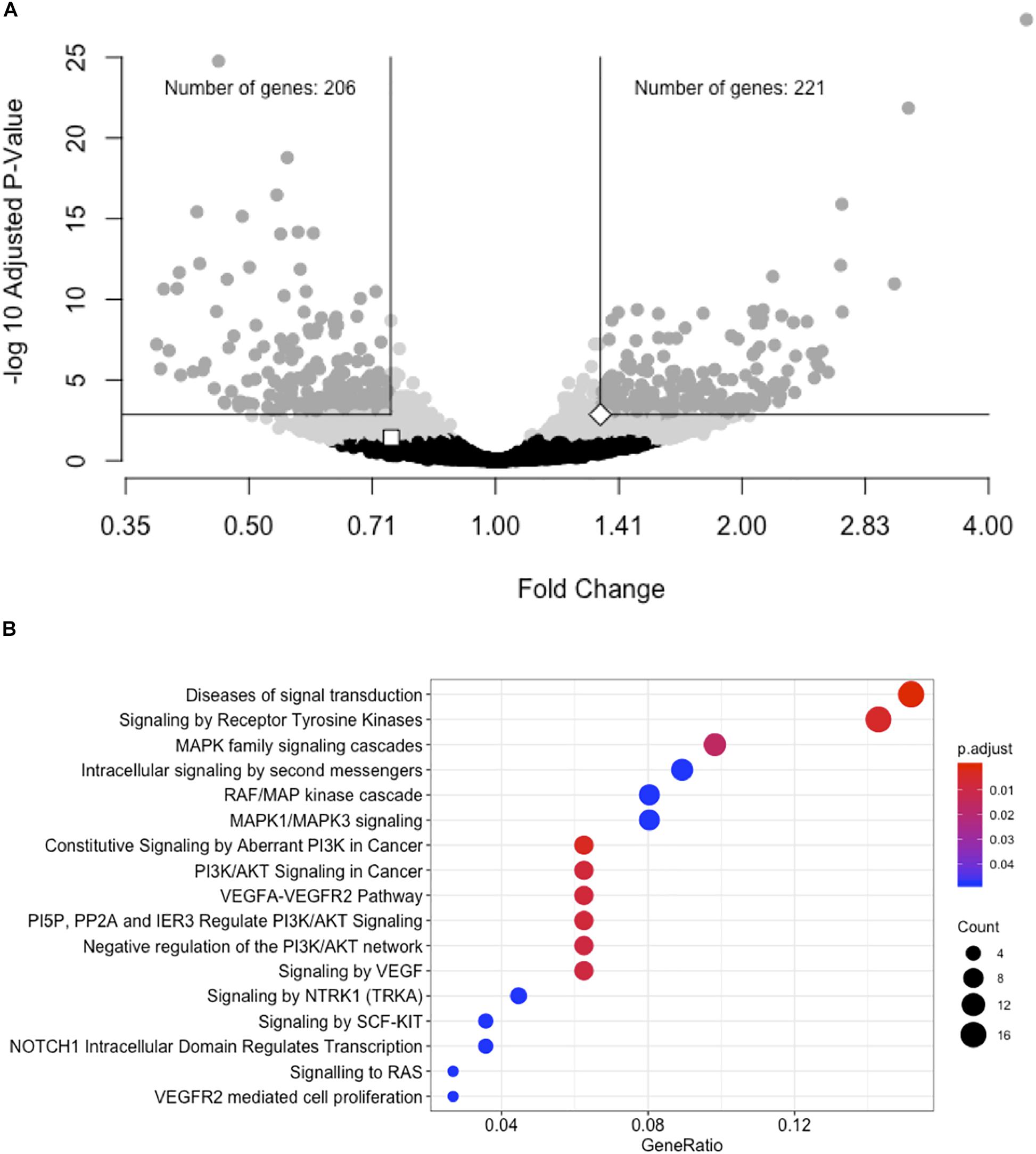
Figure 3. siRNA knockdown of LOC157273 using transcriptome-wide differential expression analysis in rs4841132 G/G genotype human hepatocytes results in significant expression changes in hundreds of transcripts, including LOC157273, PPP1R3B, and numerous putative trans targets. (A) Volcano plot showing the fold change effect of siRNA-11 and siRNA-15 knockdowns combined (X axis), compared with controls, with gray points indicating significant change in transcript expression (family-wise Padj < 0.05) (Y axis). The white square point indicates the LOC157273 transcript and shows that its knockdown reduced its expression (fold change = 0.74, P = 0.004, Padj = 0.04), as expected for a successful knockdown experiment. The white diamond point indicates the PPP1R3B transcript and shows that LOC157273 knockdown increased PPP1R3B expression 34% (fold change = 1.34, P = 4.5 × 10–5, Padj = 0.001). (B) Reactome enrichment analysis of genes with increased expression after siRNA-11 or siRNA-15 knockdown compared to controls (221 dark gray points in Panel (A); fold change > 1.34 and Padj < 0.001). Each row represents a significant Reactome pathway (family-wise P < 0.05) with GeneRatio (X axis) showing the degree to which the differentially expressed genes were enriched in the pathway. The count of differentially expressed genes within each pathway is depicted with the size of the circles, and the significance of the enrichment is depicted with the color of the circles.
Glycogen Content and Allelic Imbalance of LOC157273 Transcription in Human Hepatocytes
Patterns of LOC157273 expression and glycogen deposition in the rs4841132 A/G carrier were similar to those seen by siRNA knockdown of LOC157273: ΔCT comparing LOC157273 to GAPDH for G/G carriers averaged 8.94 (95% CI: 8.86–9.01), while the A/G carrier showed less LOC157273 lncRNA [ΔCT: 11.0 (95% CI: 9.4–12.6)]. Although we do not perform a statistical test for differences between A/G carriers and G/G carriers, we observe ∼76% decrease in expression in the A/G carrier compared to the G/G allele carriers (Table 1). Before insulin stimulation, the median glycogen store in hepatocytes from the A/G heterozygote was 0.40 nM in a first replicate and 0.44 nM in a second replicate, compared to 0.12 nM in hepatocytes from a G/G homozygote (Figure 4). Median basal glycogen concentration in two other G/G donors was 0.14 and 0.06 nM (Supplementary Figure S8). In one assay, A/G hepatocytes had median glucose concentration of 0.53 nM after insulin re-stimulation, representing a 31% increase (P = 0.004) in glycogen content over basal levels. In a replicate, glucose concentration was 0.59 nM after insulin re-stimulation, representing a consistent but non-significant increase of 33% (P = 0.06). The hepatocytes from the G/G donor had no observable increase (P = 0.7) in glycogen content over basal levels (Figure 4). To test whether the G or the A allele was responsible for the observed effects on LOC157273 action, we used a single reverse primer in exon 3 (Supplementary Table S3) to prime cDNA synthesis enriched for LOC157273 cDNA. In one assay, we observed 78% reduction of the rs4841132-A (minor) allele transcript compared to the G allele transcript. In a replicate, we observed 88% reduction (Figure 5). These results suggest that the diminished content of LOC157273 lncRNA in this A/G heterozygous hepatocyte donor results from a specific loss of lncRNA output in rs4841132-A (minor) allele carriers.
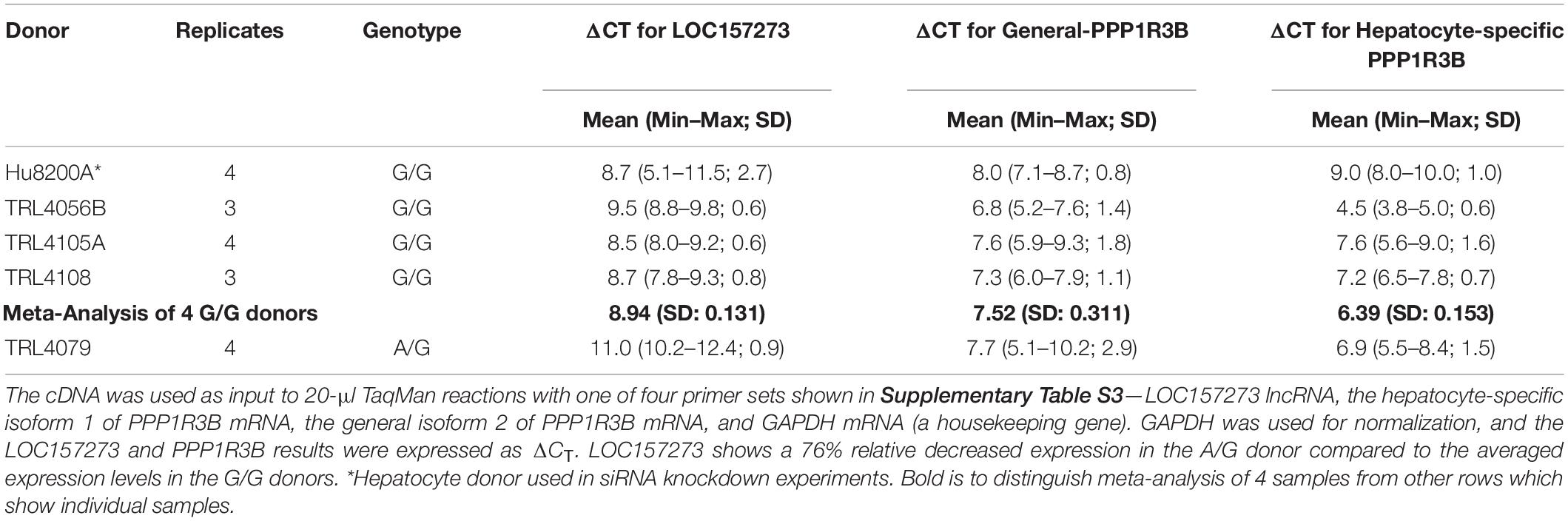
Table 1. Difference in CT (ΔCT) for target gene and GAPDH reference gene from qRT-PCR on RNA purified from primary human hepatocytes derived from four homozygous (G/G) donors and one heterozygous (A/G) donor.
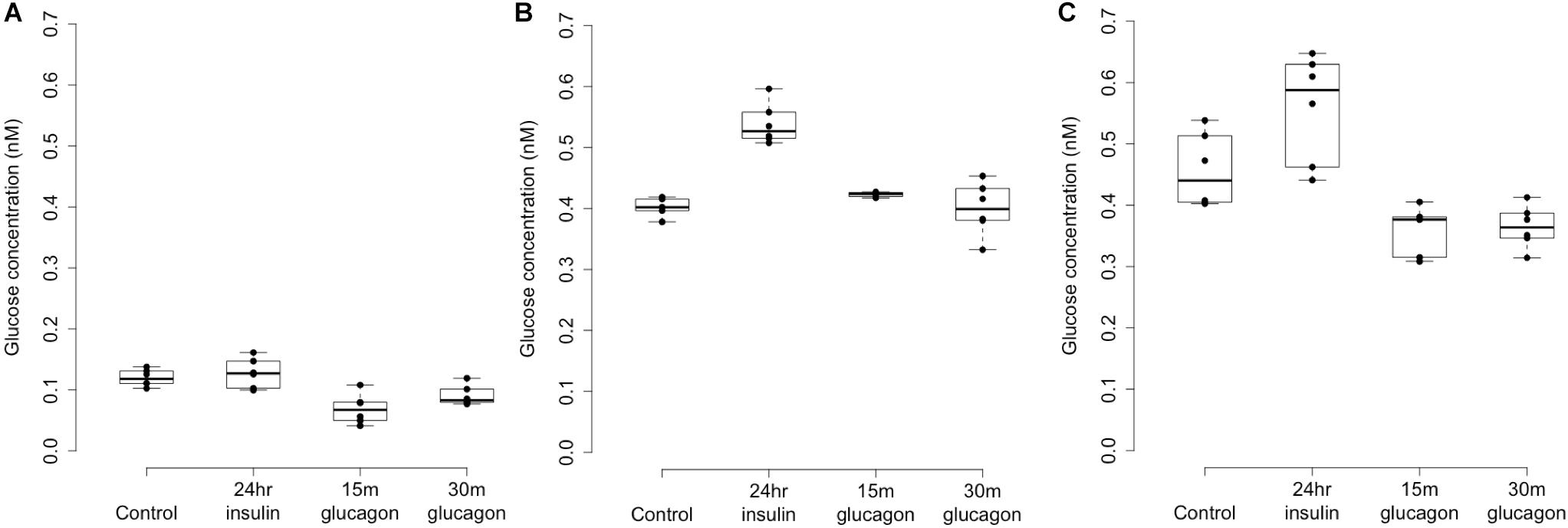
Figure 4. Glycogen deposition response after insulin re-stimulation of primary human hepatocytes. Panel (A) shows a donor with rs4841132 G/G genotype and Panels (B,C) show replicates of a donor with rs4841132 A/G genotype. Brief (15 or 30 min) treatment with 5 nM glucagon demonstrates a decrease in glycogen compared to the control, confirming that what is being measured is glycogen.
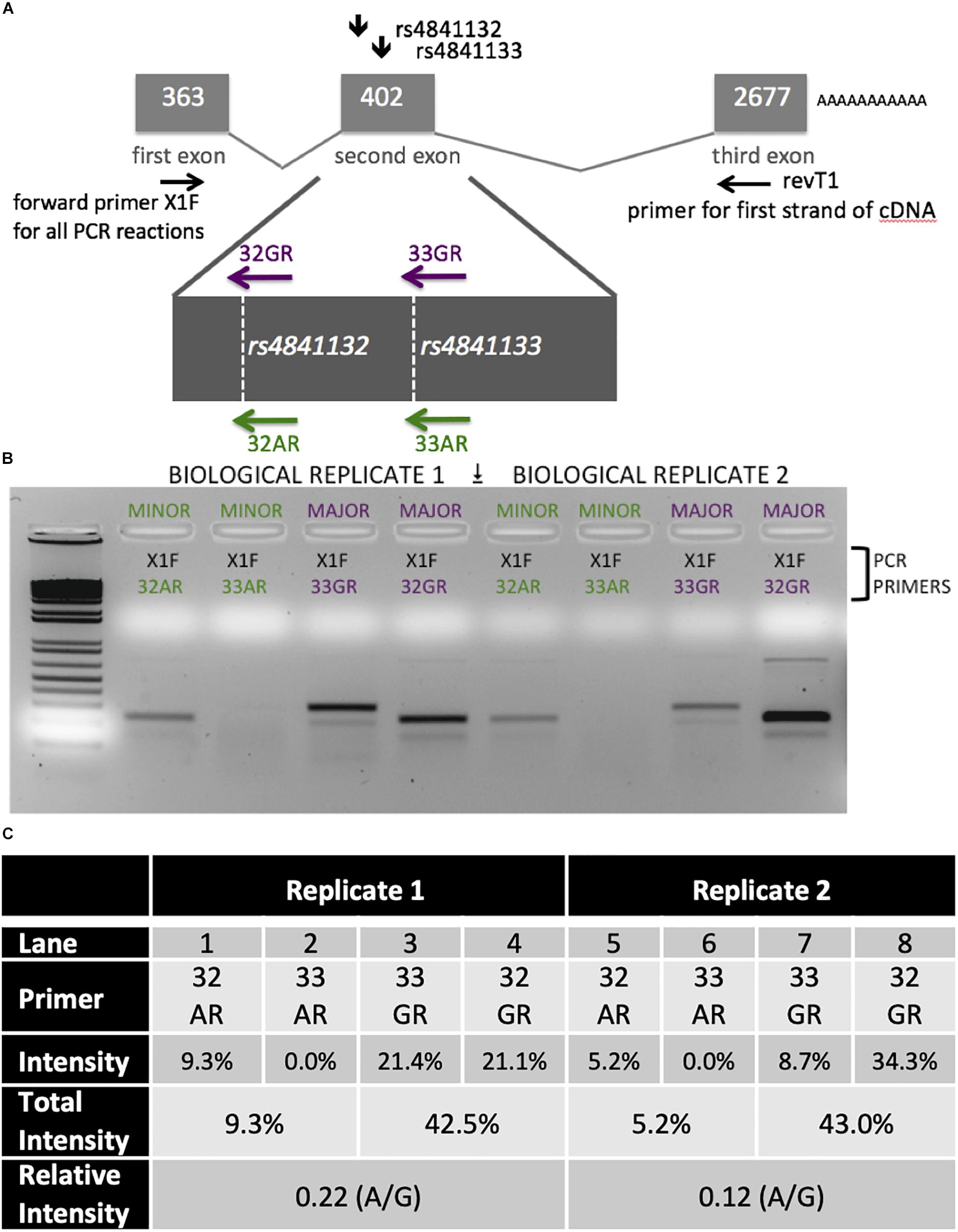
Figure 5. Analysis of LOC157273 transcription in primary hepatocytes from a heterozygous rs4841132 A/G donor demonstrates decreased transcription of the minor (A) allele. (A) RNA was transcribed into cDNA using a gene-specific strand-specific primer for two replicates. (B) Aliquots of cDNA were amplified with PCR primers seated in the first exon (black, X1F) and allele-specific primers that recognize only the major allele (magenta, 32G or 33G) or minor allele (green, 32A or 33A). The X1F-32 reaction yields a PCR product of 231 bp, while the X1F-33 reaction yields a PCR product of 302 bp. (C) Image-based quantification of the X1F-32 and X1F-33 bands from the minor allele compared to the major allele using the ImageJ software. Total intensity: sum of 32GR/33GR or 33AR/32AR lanes. Relative Intensity: quantification of the rs4841132-A minor allele intensity compared to the rs4841132-G major allele intensity.
Discussion
Over 15 years of genome-wide association discovery provides a rich abundance of loci and variants associated with T2D risk and its underlying metabolic pathophysiology. The genetic architecture of T2D is dominated by non-coding, regulatory, mostly common variation, with only ∼7% of variants convincingly shown to directly encode protein-altering mutations in Europeans (Fuchsberger et al., 2016; Mahajan et al., 2018a). The function of these hundreds of non-coding variants is being illuminated by integration of genomic association data with genomic functional information; for T2D, most data thus far come from islet chromatin regulatory mapping (Pasquali et al., 2014; van de Bunt et al., 2015; Varshney et al., 2017). Islet genomic regulatory features linked to GWAS signals include, for instance, enhancer site disruption (Roman et al., 2017), stretch enhancer potentiation (Kycia et al., 2018), and lncRNA action in human (Akerman et al., 2017) and mouse beta cell lines (Moran et al., 2012; Arnes et al., 2016).
Liver, muscle, and fat tissue also contribute to T2D pathophysiology, where progress is also being made to link human tissue-specific regulatory maps to GWAS signals (Scott et al., 2016; Pan et al., 2018). In this report, we present evidence in human liver cells that the lncRNA LOC157273 is a causal transcript at the GWAS-discovered chr8p23.1 “PPP1R3B” locus. We show that LOC157273 is expressed exclusively in human hepatocytes, is a close (<200 kb) genomic neighbor of an attractive T2D physiologic candidate gene, PPP1R3B, and is a negative regulator of PPP1R3B expression. Another nearby gene, TNKS, could also be considered a candidate causal transcript. Like PPP1R3B, loci near TKNS are associated with cardiometabolic traits, including blood pressure traits (Feitosa et al., 2018; Sung et al., 2018), and rs4841132 is an eQTL of TNKS in the liver (P = 7.9 × 10–8) according to the GTEx Portal (Ardlie et al., 2015). Unlike PPP1R3B, however, TNKS was not highly expressed in the liver, being the 5th lowest ranked tissue for TNKS expression, compared to PPP1R3B for which the liver is the 4th highest ranked tissue. Finally, there is no evidence for differential expression of TNKS after siRNA knockdown of LOC157273 (P = 0.7).
We demonstrated that LOC157273 is a predominantly cytoplasmic lncRNA. Any role it may play in the cytoplasm remains uncertain. It is unknown whether a cytoplasmic mechanism exists whereby which LOC157273 may regulate its neighbor gene PPP1R3B (presumably independent of genomic proximity). We also cannot discount an alternative role for LOC157273 in genomic regulation or a potential role for LOC157273 in dual control in both cytoplasmic and nuclear regulation of PPP1R3B.
For instance, LOC157273 might be subject to bidirectional nucleocytoplasmic shuttling with cytoplasmic excess, but still has a nuclear function in epigenetically downregulating its neighbor gene. If true, this would explain both the cytoplasmic foci (putative RNA–protein complexes allowing LOC157273 to regulate numerous genes in-trans) and why its cytoplasmic knockdown rescues PPP1R3B expression (removal of excess LOC157273, which cannot go back to the nucleus to regulate its neighbor gene in cis).
Small interfering RNA knockdown of LOC157273 nearly doubled PPP1R3B mRNA levels (vs. control) and altered the expression of other human hepatocyte transcripts. This was accompanied by an increase of over >50% in insulin-mediated hepatocyte glycogen deposition, which is an expected functional consequence of increased PPP1R3B expression. As PPP1R3B is the principal known regulator of glucose entry into the hepatic glycogen deposition pathway, its negative regulation by LOC157273 (containing rs4841132) strongly supports the lncRNA and not the protein per se as the causal transcript at the locus. Indeed, evidence for common genetic variation in PPP1R3B itself has been inconsistent for T2D risk in humans (Dunn et al., 2006; Liu et al., 2016), although rare variants in PPP1R3B are associated with human diabetes phenotypes (Abdulkarim et al., 2015; Niazi et al., 2019).
Negative regulation of PPP1R3B by LOC157273 provides partial evidence for the lncRNA to be the functional transcript at the chr8p23.1 locus. As the rs4841132-A allele occurs in about 1 in 10 people, we were able to find a single rs4841132 A/G heterozygote among all the commercial hepatocyte donors available to us. This A/G carrier had reduced LOC157273 abundance, increased PPP1R3B expression, and increased glycogen deposition vs. averages from three G/G carriers, mimicking the effect observed with knockdown in G/G hepatocytes. Although heterozygote data are from a single donor, they are concordant with other, independent data. In 125 liver biopsy samples from obese patients, the rs4841132-A allele (vs. G) was associated with higher PPP1R3B mRNA levels and reduced LOC157273 expression and protection against histologic hepatic steatosis (Dongiovanni et al., 2018). In another cohort of 1539 individuals with non-viral liver disease, the rs4841132-A allele (vs. G) was associated with increased hepatic x-ray attenuation reflecting increased glycogen deposition, consistent with a mild form of hepatic glycogenosis (Stender et al., 2018). Our data suggest that the rs4841132-A allele confers decreased transcriptional efficiency of LOC157273; reduced lncRNA abundance induces PPP1R3B upregulation as well as other transcripts apparently related to increased glycogen deposition. The large number of genes with differential expression after LOC157273 knockdown (221 upregulated and 206 downregulated) supports a scenario of LOC157273 as a master regulator of transcription in trans. Furthermore, several enriched pathways (fatty acid metabolism, PI3K/AKT signaling, among others) are concordant with enriched pathways from the transcriptome analysis of the 125 liver biopsy samples comparing carriers of the rs4841132-A allele vs. non-carriers (Dongiovanni et al., 2018). Taken together, the functional data we present support the contention that the lncRNA LOC157273 is a causal transcript at the chr8p23.1 GWAS locus, controlling T2D risk and metabolic physiology by hepatic regulation of PPP1R3B (and most likely other) transcription, thereby influencing variation in glycemia, other metabolic phenotypes, and T2D risk observed in genetic association studies.
LncRNAs as a class have diverse molecular functions, and a few lncRNAs have emerged to be associated with cardiometabolic disease (Dechamethakun and Muramatsu, 2017). MIAT (myocardial infarction associated transcript) was the first lncRNA identified by GWAS as a disease candidate gene (Ishii et al., 2006). The chromosome 9p21 lncRNA ANRIL was subsequently shown to be associated with several forms of atherosclerosis; we now know that this molecule confers protection from atherosclerosis by controlling ribosomal RNA maturation and modulating atherogenic molecular networks (Holdt et al., 2016). The regional chr9p21 GWAS signal has also been associated with T2D, but this association is not mediated by ANRIL, as the association signal for T2D is separated from that for atherosclerosis by a recombination hot spot that renders the two disease association signals in linkage equilibrium (Dauriz and Meigs, 2014). Human pancreatic islets transcribe thousands of lncRNAs, many of which are highly islet- or beta cell-specific (Moran et al., 2012), including two beta cell lncRNAs shown to cis-regulate nearby genes involved in T2D physiology. Loss-of-function screening in a human islet beta cell line identified the lncRNA PLUTO (PDX1 locus upstream transcript) as a positive regulator of PDX1, a critical transcriptional regulator of human pancreas development and beta cell function (Akerman et al., 2017). PLUTO appears to cis-regulate PDX1 by altering chromatin structure to facilitate contact between the PDX1 promoter and its enhancer cluster. Another islet-specific transcript, blinc1, has been shown to regulate the expression of groups of functionally related genes, including NKX2-2, an essential transcription factor important for beta cell developmental programs (Arnes et al., 2016).
Integrating these observations with prior evidence, we envision the following physiological model for the action of LOC157273 on T2D hepatic physiology (Figure 6). Our siRNA data suggest that LOC157273 is a functional suppressor of PPP1R3B transcription, with lower lncRNA levels associated with higher hepatocyte PPP1R3B expression. PPP1R3B is the glycogen-targeting subunit of phosphatase PP1, regulating PP1 activity by suppressing the rate at which PP1 inactivates glycogen phosphorylase (decreasing glycogen breakdown) and enhancing the rate at which it activates glycogen synthase and increases glycogen synthesis (Doherty et al., 1995). Increased liver PPP1R3B expression therefore shifts basal and insulin-stimulated hepatic glycogen flux toward storage (Gasa et al., 2000), which is consistent with our in vitro data as well as human liver imaging studies showing that the rs4841132-A allele carriers have increased hepatic attenuation on CT imaging, suggestive of increased glycogen storage (Speliotes et al., 2011; Feitosa et al., 2013). Assuming that rs4841132-A allele carriers have increased glycogen stores, we hypothesize that these will lead to reduced glucose uptake by the liver in the fasting state, consistent with observed GWAS associations with elevated fasting serum glucose and insulin levels and T2D risk (Manning et al., 2012). Additionally, abundant liver glycogen in the fasting state may be easily mobilized to glucose-6-phosphate and therefore increase glycolytic flux, consistent with observed associations with increased lactate levels in humans (Tin et al., 2016). Liver free fatty acid formation is also influenced, consistent with observed decreases in cholesterol levels (Teslovich et al., 2010). In contrast to the fasting state, in the postprandial state, the rs4841132-A allele appears to cause increased insulin-mediated glucose uptake the by the liver (Gasa et al., 2000). With liver glycogen synthesis increased, hepatic glucose uptake may increase, consistent with observed associations for SNPs near PPP1R3B with decreased 2-h post OGTT glucose levels seen in our earlier GWAS (Manning et al., 2012).
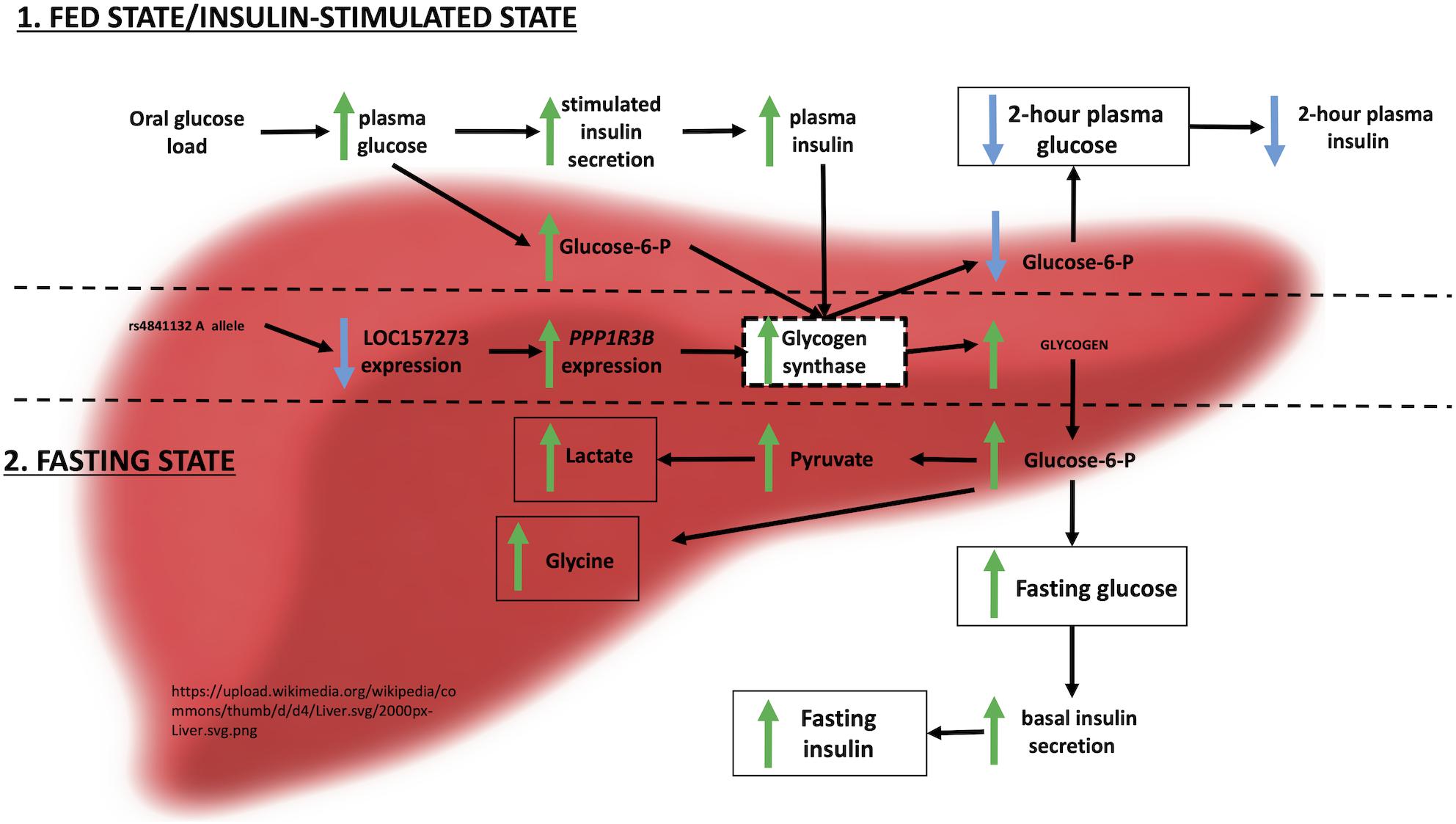
Figure 6. Physiological model of LOC157273 action on PPP1R3B, hepatic glycogen flux, and peripheral glucose and insulin levels.
Strengths of our study include a clear test of the hypothesis that LOC157273, in which the well-documented metabolic trait variant rs4841132 resides, regulates the nearby gene PPP1R3B and influences hepatocyte glycogen deposition, supporting the contention that the lncRNA is a causal transcript at the chr8p23.1 GWAS locus. That we demonstrated this in humans is important, as LOC157273 does not have an ortholog in rodents and cannot be meaningfully studied in them. Limitations include that the donors are from mixed ages, gender, and ethnic backgrounds and that we studied allelic imbalance in only one rs4841132 A/G heterozygote hepatocyte donor who was younger than the other donors, but effects in this individual were similar to lipid and glycogen hepatic storage effects seen in much larger samples with A/G carriers. Unbiased RNA-seq following siRNA knockdown of LOC157273 identified many additional transcripts that could contribute to the observed increase in hepatic glycogen deposition, but due to the small number of biological replicates available, full exploration of these signals will require future research. Finally, we do not yet know the exact molecular mechanism whereby LOC157273 regulates PPP1R3B or any of the other transcripts seen by RNA-seq. LncRNAs have multiple mechanisms that influence gene regulation; here, we might postulate epigenetic and/or cytoplasmic mechanisms to explain both its cytoplasmic location and apparently broad transcriptional effects (Font-Cunill et al., 2018).
Causal transcripts, relevant tissues, and molecular mechanisms underlying the hundreds of T2D-associated genomic loci are now coming to light. A goal of modern chronic disease genomics is to identify new mechanisms for therapeutic targeting. RNA therapeutics is one novel frontier for genomic medicine. siRNA therapeutics, which as in our approach loads its target RNA into the endogenous cytoplasmic RNA-induced silencing complex (RISC), are now in late-stage clinical trials for lipid-lowering through PCSK9 inhibition (Levin, 2019). The liver is especially amenable to RNA therapeutics; glycemic control via siRNA regulation of glycogen entry into the liver arises as a tantalizing possibility. However, the mild glycogenosis that appears to accompany genetic variation at rs4841132 raises the possibility that lowering blood glucose by increasing hepatic storage of glycogen may have its own risks (Stender et al., 2018). While our data support a genetic regulatory relationship between LOC157273 and PPP1R3B, LOC157273 appears to regulate many other transcripts whose action in regulating hepatic glycogen and cholesterol flux remain to be explained. Nonetheless, we have identified a lncRNA to be a causal transcript at a hepatic T2D-cardiometabolic disease GWAS locus, opening the window to the possibility of new, RNA-based therapeutic pathways for therapy and prevention of T2D.
Data Availability Statement
The datasets generated for this study can be found in the NCBI GEO repository: https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE142385.
Author Contributions
JM and LL led the study and provided funding and material support. AM, AG, EK, RS, JM, and LL wrote the initial draft and submitted the final draft of manuscript. AG, PT, JC, and DJ conducted the laboratory experiments. AM, EK, and JB conducted statistical analysis and bioinformatics. All authors interpreted the data, provided the intellectual input, reviewed the drafts of the manuscript, and approved the final version of the manuscript.
Funding
AM was supported by K01 DK107836. RS was supported by U01 DK078616. JM was supported by R01 DK078616, U01 DK078616, K24 DK080140, and an American Diabetes Association Mentored Scientist Career Development Award. LL was supported by the NIH Director’s New Innovator Award 1DP2-CA196375 and by the Wayne State University 2018–2019 Charles H. Gershenson Distinguished Faculty Fellowship. MG was supported by the Eris M. Field Chair in Diabetes Research. JR was supported in part by R01 DK078616. MG and JR were supported by NIH National Institute of Diabetes and Digestive and Kidney Disease Diabetes Research Center (DRC) grant P30 DK063491 to the Southern California Diabetes Endocrinology Research Center and NIH National Center for Advancing Translational Science (NCATS) UCLA CTSI Grant Number UL1TR001881. MU was supported by NIDDK K23DK114551. JM is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fgene.2020.00615/full#supplementary-material
Footnotes
References
Abdulkarim, B., Nicolino, M., Igoillo-Esteve, M., Daures, M., Romero, S., Philippi, A., et al. (2015). A missense mutation in PPP1R15B causes a syndrome including diabetes, short stature, and microcephaly. Diabetes 64, 3951–3962.
Agius, L. (2015). Role of glycogen phosphorylase in liver glycogen metabolism. Mol Aspects Med. 46, 34–45. doi: 10.1016/j.mam.2015.09.002
Akerman, I., Tu, Z., Beucher, A., Rolando, D. M. Y., Sauty-Colace, C., Benazra, M., et al. (2017). Human pancreatic beta cell lncRNAs control cell-specific regulatory networks. Cell Metab. 25, 400–411. doi: 10.1016/j.cmet.2016.11.016
Anders, S., and Huber, W. (2010). Differential expression analysis for sequence count data. Genome Biol. 11:R106.
Ardlie, K. G., Deluca, D. S., Segrè, A. V., Sullivan, T. J., Young, T. R., Gelfand, E. T., et al. (2015). Human genomics. The genotype-tissue expression (GTEx) pilot analysis: multitissue gene regulation in humans. Science 348, 648–660.
Arnes, L., Akerman, I., Balderes, D. A., Ferrer, J., and Sussel, L. (2016). βlinc1 encodes a long noncoding RNA that regulates islet beta-cell formation and function. Genes Dev. 30, 502–507. doi: 10.1101/gad.273821.115
Block, G. D., Locker, J., Bowen, W. C., Petersen, B. E., Katyal, S., Strom, S. C., et al. (1996). Population expansion, clonal growth, and specific differentiation patterns in primary cultures of hepatocytes induced by HGF/SF, EGF and TGF alpha in a chemically defined (HGM) medium. J. Cell Biol. 132, 1133–1149. doi: 10.1083/jcb.132.6.1133
Cauchi, S., Nead, K. T., Choquet, H., Horber, F., Potoczna, N., Balkau, B., et al. (2008). The genetic susceptibility to type 2 diabetes may be modulated by obesity status: implications for association studies. BMC Med. Genet. 9:45. doi: 10.1186/1471-2350-9-45
Cohen, P. (2006). The twentieth century struggle to decipher insulin signalling. Na. Rev. Mol. Cell Biol. 7, 867–873. doi: 10.1038/nrm2043
Dauriz, M., and Meigs, J. B. (2014). Current insights into the joint genetic basis of Type 2 diabetes and coronary heart disease. Curr. Cardiovas. Risk Rep. 8:368.
Dechamethakun, S., and Muramatsu, M. (2017). Long noncoding RNA variations in cardiometabolic diseases. J. Hum. Genet. 62, 97–104. doi: 10.1038/jhg.2016.70
Doherty, M. J., Moorhead, G., Morrice, N., Cohen, P., and Cohen, P. T. (1995). Amino acid sequence and expression of the hepatic glycogen-binding (GL)-subunit of protein phosphatase-1. FEBS Lett. 375, 294–298. doi: 10.1016/0014-5793(95)01184-g
Dongiovanni, P., Meroni, M., Mancina, R. M., Baselli, G., Rametta, R., Pelusi, S., et al. (2018). Protein phosphatase 1 regulatory subunit 3B gene variation protects against hepatic fat accumulation and fibrosis in individuals at high risk of nonalcoholic fatty liver disease. Hepatol. Commun. 2, 666–675. doi: 10.1002/hep4.1192
Dunn, J. S., Mlynarski, W. M., Pezzolesi, M. G., Borowiec, M., Powers, C., Krolewski, A. S., et al. (2006). Examination of PPP1R3B as a candidate gene for the type 2 diabetes and MODY loci on chromosome 8p23. Ann. Hum. Genet. 70(Pt 5), 587–593. doi: 10.1111/j.1469-1809.2005.00248.x
Engstrom, P. G., Suzuki, H., Ninomiya, N., Akalin, A., Sessa, L., Lavorgna, G., et al. (2006). Complex Loci in human and mouse genomes. PLoS Genet. 2:e47. doi: 10.1371/journal.pgen.0020047
Feitosa, M. F., Kraja, A. T., Chasman, D. I., Sung, Y. J., Winkler, T. W., Ntalla, I., et al. (2018). Novel genetic associations for blood pressure identified via gene-alcohol interaction in up to 570K individuals across multiple ancestries. PLoS One 13:e0198166. doi: 10.1371/journal.pone.0198166
Feitosa, M. F., Wojczynski, M. K., North, K. E., Zhang, Q., Province, M. A., Carr, J. J., et al. (2013). The ERLIN1-CHUK-CWF19L1 gene cluster influences liver fat deposition and hepatic inflammation in the NHLBI family heart study. Atherosclerosis 228, 175–180. doi: 10.1016/j.atherosclerosis.2013.01.038
Font-Cunill, B., Arnes, L., Ferrer, J., Sussel, L., and Beucher, A. (2018). Long non-coding RNAs as local regulators of pancreatic islet transcription factor genes. Front. Genet. 9:524. doi: 10.3389/fgene.2018.00524
Forrest, A. R., Kawaji, H., Rehli, M., Baillie, J. K., de Hoon, M. J., Haberle, V., et al. (2014). A promoter-level mammalian expression atlas. Nature 507, 462–470. doi: 10.1038/nature13182
Fuchsberger, C., Flannick, J., Teslovich, T. M., Mahajan, A., Agarwala, V., Gaulton, K. J., et al. (2016). The genetic architecture of type 2 diabetes. Nature 536, 41–47.
Gasa, R., Clark, C., Yang, R., DePaoli-Roach, A. A., and Newgard, C. B. (2002). Reversal of diet-induced glucose intolerance by hepatic expression of a variant glycogen-targeting subunit of protein phosphatase-1. J. Biol. Chem. 277, 1524–1530. doi: 10.1074/jbc.m107744200
Gasa, R., Jensen, P. B., Berman, H. K., Brady, M. J., DePaoli-Roach, A. A., and Newgard, C. B. (2000). Distinctive regulatory and metabolic properties of glycogen-targeting subunits of protein phosphatase-1 (PTG, GL, GM/RGl) expressed in hepatocytes. J. Biol. Chem. 275, 26396–26403. doi: 10.1074/jbc.m002427200
Gómez-Lechón, M. J., Ponsoda, X., and Castell, J. V. (1996). A microassay for measuring glycogen in 96-well-cultured cells. Anal. Biochem. 236, 296–301. doi: 10.1006/abio.1996.0170
Hayes, M. G., Urbanek, M., Hivert, M. F., Armstrong, L. L., Morrison, J., Guo, C., et al. (2013). Identification of HKDC1 and BACE2 as genes influencing glycemic traits during pregnancy through genome-wide association studies. Diabetes 62, 3282–3291. doi: 10.2337/db12-1692
Holdt, L. M., Stahringer, A., Sass, K., Pichler, G., Kulak, N. A., Wilfert, W., et al. (2016). Circular non-coding RNA ANRIL modulates ribosomal RNA maturation and atherosclerosis in humans. Na. t Commun. 7:12429.
Inouye, M., Ripatti, S., Kettunen, J., Lyytikainen, L. P., Oksala, N., Laurila, P. P., et al. (2012). Novel Loci for metabolic networks and multi-tissue expression studies reveal genes for atherosclerosis. PLoS Genet. 8:e1002907. doi: 10.1371/journal.pgen.1002907
Ishii, N., Ozaki, K., Sato, H., Mizuno, H., Saito, S., Takahashi, A., et al. (2006). Identification of a novel non-coding RNA, MIAT, that confers risk of myocardial infarction. J. Hum. Genet. 51, 1087–1099. doi: 10.1007/s10038-006-0070-9
Katayama, S., Tomaru, Y., Kasukawa, T., Waki, K., Nakanishi, M., Nakamura, M., et al. (2005). Antisense transcription in the mammalian transcriptome. Science 309, 1564–1566. doi: 10.1126/science.1112009
Knowler, W. C., Pettitt, D. J., Savage, P. J., and Bennett, P. H. (1981). Diabetes incidence in Pima indians: contributions of obesity and parental diabetes. Am. J. Epidemiol. 113, 144–156. doi: 10.1093/oxfordjournals.aje.a113079
Kycia, I., Wolford, B. N., Huyghe, J. R., Fuchsberger, C., Vadlamudi, S., Kursawe, R., et al. (2018). A common Type 2 diabetes risk variant potentiates activity of an evolutionarily conserved islet stretch enhancer and increases C2CD4A and C2CD4B expression. Am. J. Hum. Genet. 102, 620–635. doi: 10.1016/j.ajhg.2018.02.020
Lettre, G., Palmer, C. D., Young, T., Ejebe, K. G., Allayee, H., Benjamin, E. J., et al. (2011). Genome-wide association study of coronary heart disease and its risk factors in 8,090 African Americans: the NHLBI CARe Project. PLoS Genet. 7:e1001300. doi: 10.1371/journal.pgen.1001300
Levin, A. A. (2019). Treating disease at the RNA level with oligonucleotides. N. Engl. J. Med. 380, 57–70. doi: 10.1056/nejmra1705346
Ligthart, S., de Vries, P. S., Uitterlinden, A. G., Hofman, A., Franco, O. H., Chasman, D. I., et al. (2015). Pleiotropy among common genetic loci identified for cardiometabolic disorders and C-reactive protein. PLoS One 10:e0118859. doi: 10.1371/journal.pone.0118859
Liu, C. T., Raghavan, S., Maruthur, N., Kabagambe, E. K., Hong, J., Ng, M. C., et al. (2016). Trans-ethnic meta-analysis and functional annotation illuminates the genetic architecture of fasting glucose and insulin. Am. J. Hum. Genet. 99, 56–75.
Mahajan, A., Taliun, D., Thurner, M., Robertson, N. R., Torres, J. M., Rayner, N. W., et al. (2018a). Fine-mapping type 2 diabetes loci to single-variant resolution using high-density imputation and islet-specific epigenome maps. Nat. Genet. 50, 1505–1513.
Mahajan, A., Wessel, J., Willems, S. M., Zhao, W., Robertson, N. R., Chu, A. Y., et al. (2018b). Refining the accuracy of validated target identification through coding variant fine-mapping in type 2 diabetes. Nat. Genet. 50, 559–571.
Manning, A. K., Hivert, M. F., Scott, R. A., Grimsby, J. L., Bouatia-Naji, N., Chen, H., et al. (2012). A genome-wide approach accounting for body mass index identifies genetic variants influencing fasting glycemic traits and insulin resistance. Nat. Genet. 44, 659–669.
McCarthy, D. J., Chen, Y., and Smyth, G. K. (2012). Differential expression analysis of multifactor RNA-Seq experiments with respect to biological variation. Nucleic Acids Res. 40, 4288–4297. doi: 10.1093/nar/gks042
Mitchel, K., Theusch, E., Cubitt, C., Dose, A. C., Stevens, K., Naidoo, D., et al. (2016). RP1-13D10.2 Is a novel modulator of statin-induced changes in cholesterol. Circ. Cardiovasc. Genet. 9, 223–230. doi: 10.1161/circgenetics.115.001274
Moran, I., Akerman, I., van de Bunt, M., Xie, R., Benazra, M., Nammo, T., et al. (2012). Human beta cell transcriptome analysis uncovers lncRNAs that are tissue-specific, dynamically regulated, and abnormally expressed in type 2 diabetes. Cell Metab. 16, 435–448. doi: 10.1016/j.cmet.2012.08.010
Narayan, K. M., Boyle, J. P., Geiss, L. S., Saaddine, J. B., and Thompson, T. J. (2006). Impact of recent increase in incidence on future diabetes burden: U.S., 2005-2050. Diabetes Care 29, 2114–2116. doi: 10.2337/dc06-1136
Newgard, C. B., Brady, M. J., O’Doherty, R. M., and Saltiel, A. R. (2000). Organizing glucose disposal: emerging roles of the glycogen targeting subunits of protein phosphatase-1. Diabetes 49, 1967–1977. doi: 10.2337/diabetes.49.12.1967
Niazi, R. K., Sun, J., Have, C. T., Hollensted, M., Linneberg, A., Pedersen, O., et al. (2019). Increased frequency of rare missense PPP1R3B variants among Danish patients with type 2 diabetes. PLoS One 14:e0210114. doi: 10.1371/journal.pone.0210114
Orom, U. A., Derrien, T., Beringer, M., Gumireddy, K., Gardini, A., Bussotti, G., et al. (2010). Long noncoding RNAs with enhancer-like function in human cells. Cell 143, 46–58. doi: 10.1016/j.cell.2010.09.001
Pan, D. Z., Garske, K. M., Alvarez, M., Bhagat, Y. V., Boocock, J., Nikkola, E., et al. (2018). Integration of human adipocyte chromosomal interactions with adipose gene expression prioritizes obesity-related genes from GWAS. Nat. Commun. 9:1512.
Pasquali, L., Gaulton, K. J., Rodriguez-Segui, S. A., Mularoni, L., Miguel-Escalada, I., Akerman, I., et al. (2014). Pancreatic islet enhancer clusters enriched in type 2 diabetes risk-associated variants. Nat. Genet. 46, 136–143. doi: 10.1038/ng.2870
Raffield, L. M., Louie, T., Sofer, T., Jain, D., Ipp, E., Taylor, K. D., et al. (2017). Genome-wide association study of iron traits and relation to diabetes in the Hispanic Community Health Study/Study of Latinos (HCHS/SOL): potential genomic intersection of iron and glucose regulation? Hum. Mol. Genet. 26, 1966–1978. doi: 10.1093/hmg/ddx082
Roman, T. S., Cannon, M. E., Vadlamudi, S., Buchkovich, M. L., Wolford, B. N., Welch, R. P., et al. (2017). A type 2 diabetes-associated functional regulatory variant in a pancreatic islet enhancer at the ADCY5 Locus. Diabetes 66, 2521–2530. doi: 10.2337/db17-0464
Scott, L. J., Erdos, M. R., Huyghe, J. R., Welch, R. P., Beck, A. T., Wolford, B. N., et al. (2016). The genetic regulatory signature of type 2 diabetes in human skeletal muscle. Nat. Commun. 7:11764.
Speliotes, E. K., Yerges-Armstrong, L. M., Wu, J., Hernaez, R., Kim, L. J., Palmer, C. D., et al. (2011). Genome-wide association analysis identifies variants associated with nonalcoholic fatty liver disease that have distinct effects on metabolic traits. PLoS Genet. 7:e1001324. doi: 10.1371/journal.pgen.1001324
Stender, S., Smagris, E., Lauridsen, B. K., Kofoed, K. F., Nordestgaard, B. G., Tybjaerg-Hansen, A., et al. (2018). Relationship between genetic variation at PPP1R3B and levels of liver glycogen and triglyceride. Hepatology 67, 2182–2195. doi: 10.1002/hep.29751
Sung, Y. J., Winkler, T. W., de Las Fuentes, L., Bentley, A. R., Brown, M. R., Kraja, A. T., et al. (2018). A large-scale multi-ancestry genome-wide study accounting for smoking behavior identifies multiple significant loci for blood Pressure. Am. J. Hum. Genet 102, 375–400.
Tajbakhsh, A., Khorrami, M. S., Hassanian, S. M., Aghasizade, M., Pasdar, A., Maftouh, M., et al. (2016). The 9p21 locus and its potential role in atherosclerosis susceptibility. Molecular mechanisms and clinical implications. Curr. Pharm. Des. 22, 5730–5737. doi: 10.2174/1381612822666160628082453
Teslovich, T. M., Musunuru, K., Smith, A. V., Edmondson, A. C., Stylianou, I. M., Koseki, M., et al. (2010). Biological, clinical and population relevance of 95 loci for blood lipids. Nature 466, 707–713.
Tin, A., Balakrishnan, P., Beaty, T. H., Boerwinkle, E., Hoogeveen, R. C., Young, J. H., et al. (2016). GCKR and PPP1R3B identified as genome-wide significant loci for plasma lactate: the atherosclerosis risk in communities (ARIC) study. Diabet Med. 33, 968–975. doi: 10.1111/dme.12971
van de Bunt, M., Manning Fox, J. E., Dai, X., Barrett, A., Grey, C., Li, L., et al. (2015). Transcript expression data from human islets links regulatory signals from genome-wide association studies for Type 2 diabetes and glycemic traits to their downstream effectors. PLoS Genet. 11:e1005694. doi: 10.1371/journal.pgen.1005694
Varshney, A., Scott, L. J., Welch, R. P., Erdos, M. R., Chines, P. S., Narisu, N., et al. (2017). Genetic regulatory signatures underlying islet gene expression and type 2 diabetes. Proc. Natl. Acad. Sci. U.S.A. 114, 2301–2306. doi: 10.1073/pnas.1621192114
Wessel, J., Chu, A. Y., Willems, S. M., Wang, S., Yaghootkar, H., Brody, J. A., et al. (2015). Low-frequency and rare exome chip variants associate with fasting glucose and type 2 diabetes susceptibility. Nat. Commun. 6:5897.
Willer, C. J., Schmidt, E. M., Sengupta, S., Peloso, G. M., Gustafsson, S., Kanoni, S., et al. (2013). Discovery and refinement of loci associated with lipid levels. Nat. Genet. 45, 1274–1283. doi: 10.1038/ng.2797
Wood, E. J., Chin-Inmanu, K., Jia, H., and Lipovich, L. (2013). Sense-antisense gene pairs: sequence, transcription, and structure are not conserved between human and mouse. Front. Genet. 4:183. doi: 10.3389/fgene.2013.00183
Wu, H., Wang, C., and Wu, Z. (2013). A new shrinkage estimator for dispersion improves differential expression detection in RNA-seq data. Biostatistics 14, 232–243. doi: 10.1093/biostatistics/kxs033
Keywords: insulin resistance, hepatic glycogen storage, long non-coding RNA, metabolism, type 2 diabetes, regulatory mechanisms
Citation: Manning AK, Goustin AS, Kleinbrink EL, Thepsuwan P, Cai J, Ju D, Leong A, Udler MS, Brown JB, Goodarzi MO, Rotter JI, Sladek R, Meigs JB and Lipovich L (2020) A Long Non-coding RNA, LOC157273, Is an Effector Transcript at the Chromosome 8p23.1-PPP1R3B Metabolic Traits and Type 2 Diabetes Risk Locus. Front. Genet. 11:615. doi: 10.3389/fgene.2020.00615
Received: 20 December 2019; Accepted: 20 May 2020;
Published: 10 July 2020.
Edited by:
Veronique Vitart, University of Edinburgh, United KingdomReviewed by:
Tony Merriman, University of Otago, New ZealandSamuel Lukowski, Boehringer Ingelheim RCV GmbH & Co KG, Austria
Copyright © 2020 Manning, Goustin, Kleinbrink, Thepsuwan, Cai, Ju, Leong, Udler, Brown, Goodarzi, Rotter, Sladek, Meigs and Lipovich. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: James B. Meigs, JMEIGS@mgh.harvard.edu; Leonard Lipovich, LLipovich@med.wayne.edu
†These authors have contributed equally to this work
‡ORCID: James B. Meigs, orcid.org/0000-0002-2439-2657; Leonard Lipovich, orcid.org/0000-0002-0531-3570
 Alisa K. Manning
Alisa K. Manning Anton Scott Goustin
Anton Scott Goustin Erica L. Kleinbrink
Erica L. Kleinbrink Pattaraporn Thepsuwan4
Pattaraporn Thepsuwan4 James Bentley Brown
James Bentley Brown Jerome I. Rotter
Jerome I. Rotter Robert Sladek
Robert Sladek Leonard Lipovich
Leonard Lipovich