Abstract
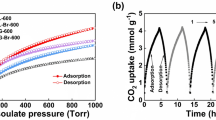
Safely and highly selective acetylene (C2H2) capture is a great challenge, because of its highly explosive nature, as well as its nearly similar molecule size and boiling point toward the main impurity of carbon dioxide (CO2). Adsorption separation has shown a promising future. Herein, a new nanoporous coordination polymer (PCP) adsorbent with fixed and free Cu ions (termed NTU-66-Cu) was prepared through post-synthetic approach via cation exchanging from the pristine NTU-66, an anionic framework with new 3, 4, 6-c topology and two kinds of cages. The NTU-66-Cu shows significantly improved C2H2/CO2 selectivity from 6 to 32 (v/v: 1/1) or 4 to 42 (v/v: 1/4) at low pressure under 298 K, along with enhanced C2H2 capacity (from 89.22 to 111.53 cm3·g−1). More importantly, this observation was further validated by density functional theory (DFT) calculations and breakthrough experiments under continuous and dynamic conditions. Further, the excellent chemical stability enables this adsorbent to achieve recycle C2H2/CO2 separation without loss of C2H2 capacity.

Similar content being viewed by others
References
Pässler, P.; Hefner, W.; Buckl, K.; Meinass, H.; Meiswinkel, A.; Wernicke, H.-J.; Ebersberg, G.; Müller, R.; Bässler, J.; Behringer, H. et al. Acetylene. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH: Weinheim, Germany, 2000.
Stang, P. J.; Diederich, F. Modern Acetylene Chemistry; VCH: New York, 1995.
Reid, C. R.; Thomas, K. M. Adsorption kinetics and size exclusion properties of probe molecules for the selective porosity in a carbon molecular sieve used for air separation. J. Phys. Chem. B2001, 105, 10619–10629.
Lin, J. Y. S. Molecular sieves for gas separation. Science2016, 353, 121–122.
Sholl, D. S.; Lively, R. P. Seven chemical separations to change the world. Nature2016, 532, 435–437.
Wang, H.; Li, J. Microporous metal-organic frameworks for adsorptive separation of C5–C6 alkane isomers. Acc. Chem. Res.2019, 52, 1968–1978.
Furukawa, H.; Cordova, K. E.; O’Keeffe, M.; Yaghi, O. M. The chemistry and applications of metal-organic frameworks. Science2013, 341, 1230444.
Walton, K. S. Metal-organic frameworks: Recognizing the unrecognizable. Nat. Chem.2014, 6, 277–278.
Yang, S. H.; Ramirez-Cuesta, A. J.; Newby, R.; Garcia-Sakai, V.; Manuel, P.; Callear, S. K.; Campbell, S. I.; Tang, C. C.; Schröder, M. Supramolecular binding and separation of hydrocarbons within a functionalized porous metal-organic framework. Nat. Chem.2015, 7, 121–129.
McDonald, T. M.; Mason, J. A.; Kong, X. Q.; Bloch, E. D.; Gygi, D.; Dani, A.; Crocella, V.; Giordanino, F.; Odoh, S. O.; Drisdell, W. S. et al. Cooperative insertion of CO2 in diamine-appended metal-organic frameworks. Nature2015, 519, 303–308.
Cadiau, A.; Belmabkhout, Y.; Adil, K.; Bhatt, P. M.; Pillai, R. S.; Shkurenko, A.; Martineau-Corcos, C.; Maurin, G.; Eddaoudi, M. Hydrolytically stable fluorinated metal-organic frameworks for energy-efficient dehydration. Science2017, 356, 731–735.
Liao, P. Q.; Huang, N. Y.; Zhang, W. X.; Zhang, J. P.; Chen, X. M. Controlling guest conformation for efficient purification of butadiene. Science2017, 356, 1193–1196.
Jiang, J. J.; Lu, Z. Y.; Zhang, M. X.; Duan, J. G.; Zhang, W. W.; Pan, Y.; Bai, J. F. Higher symmetry multinuclear clusters of metal-organic frameworks for highly selective CO2 capture. J. Am. Chem. Soc.2018, 140, 17825–17829.
Wu, H. H.; Gong, Q. H.; Olson, D. H.; Li, J. Commensurate adsorption of hydrocarbons and alcohols in microporous metal organic frameworks. Chem. Rev.2012, 112, 836–868.
Duan, J. G.; Jin, W. Q.; Kitagawa, S. Water-resistant porous coordination polymers for gas separation. Coord. Chem. Rev.2017, 332, 48–74.
Qazvini, O. T.; Babarao, R.; Telfer, S. G. Multipurpose metal-organic framework for the adsorption of acetylene: Ethylene purification and carbon dioxide removal. Chem. Mater.2019, 31, 4919–4926.
Han, Y.; Li, J. R.; Xie, Y. B.; Guo, G. S. Substitution reactions in metal-organic frameworks and metal-organic polyhedra. Chem. Soc. Rev.2014, 43, 5952–5981.
Sen, R.; Saha, D.; Koner, S.; Brandao, P.; Lin, Z. Single crystal to single crystal (SC-to-SC) transformation from a nonporous to porous metal-organic framework and its application potential in gas adsorption and Suzuki coupling reaction through postmodification. Chem.—Eur. J.2015, 21, 5962–5971.
Li, L.; da Silva, I.; Kolokolov, D. I.; Han, X.; Li, J. N.; Smith, G.; Cheng, Y. Q.; Daemen, L. L.; Morris, C. G.; Godfrey, H. G. W. et al. Post-synthetic modulation of the charge distribution in a metal-organic framework for optimal binding of carbon dioxide and sulfur dioxide. Chem. Sci.2019, 10, 1472–1482.
Fracaroli, A. M.; Siman, P.; Nagib, D. A.; Suzuki, M.; Furukawa, H.; Toste, F. D.; Yaghi, O. M. Seven post-synthetic covalent reactions in tandem leading to enzyme-like complexity within metal-organic framework crystals. J. Am. Chem. Soc.2016, 138, 8352–8355.
Wang, X. J.; Li, P. Z.; Liu, L.; Zhang, Q.; Borah, P.; Wong, J. D.; Chan, X. X.; Rakesh, G.; Li, Y. X.; Zhao, Y. L. Significant gas uptake enhancement by post-exchange of zinc(II) with copper(II) within a metal-organic framework. Chem. Commun.2012, 48, 10286–10288.
Patel, H. A.; Islamoglu, T.; Liu, Z. C.; Nalluri, S. K. M.; Samanta, A.; Anamimoghadam, O.; Malliakas, C. D.; Farha, O. K.; Stoddart, J. F. Noninvasive substitution of K+ sites in cyclodextrin metal-organic frameworks by Li+ ions. J. Am. Chem. Soc.2017, 139, 11020–11023.
Deng, M. L.; Pan, Y.; Zhu, J. X.; Chen, Z. X.; Sun, Z. Z.; Sun, J. Y.; Ling, Y.; Zhou, Y. M.; Feng, P. Y. Cation-exchange approach to tuning the flexibility of a metal-organic framework for gated adsorption. Inorg. Chem.2017, 56, 5069–5075.
Gong, Y. N.; Meng, M.; Zhong, D. C.; Huang, Y. L.; Jiang, L.; Lu, T. B. Counter-cation modulation of hydrogen and methane storage in a sodalite-type porous metal-organic framework. Chem. Commun.2012, 48, 12002–12004.
Yang, S. H.; Lin, X.; Blake, A. J.; Walker, G. S.; Hubberstey, P.; Champness, N. R.; Schroder, M. Cation-induced kinetic trapping and enhanced hydrogen adsorption in a modulated anionic metal-organic framework. Nat. Chem.2009, 1, 487–493.
Nouar, F.; Eckert, J.; Eubank, J. F.; Forster, P.; Eddaoudi, M. Zeolite-like metal-organic frameworks (ZMOFs) as hydrogen storage platform: Lithium and magnesium ion-exchange and H2-(rho-ZMOF) interaction studies. J. Am. Chem. Soc.2009, 131, 2864–2870.
An, J.; Rosi, N. L. Tuning MOF CO2 adsorption properties via cation exchange. J. Am. Chem. Soc.2010, 132, 5578–5579.
Matsuda, R.; Kitaura, R.; Kitagawa, S.; Kubota, Y.; Belosludov, R. V.; Kobayashi, T. C.; Sakamoto, H.; Chiba, T.; Takata, M.; Kawazoe, Y. et al. Highly controlled acetylene accommodation in a metal-organic microporous material. Nature2005, 436, 238–241.
Duan, J. G.; Higuchi, M.; Zheng, J. J.; Noro, S. I.; Chang, I. Y.; Hyeon-Deuk, K.; Mathew, S.; Kusaka, S.; Sivaniah, E.; Matsuda, R. et al. Density gradation of open metal sites in the mesospace of porous coordination polymers. J. Am. Chem. Soc.2017, 139, 11576–11583.
Lyu, H.; Zhang, Q.; Wang, Y.; Duan, J. G. Unified meso-pores and dense Cu2+ sites in porous coordination polymers for highly efficient gas storage and separation. Dalton Trans.2018, 47, 4424–4427.
Foo, M. L.; Matsuda, R.; Hijikata, Y.; Krishna, R.; Sato, H.; Horike, S.; Hori, A.; Duan, J. G.; Sato, Y.; Kubota, Y. et al. An adsorbate discriminatory gate effect in a flexible porous coordination polymer for selective adsorption of CO2 over C2H2. J. Am. Chem. Soc.2016, 138, 3022–3030.
Duan, J. G.; Yang, Z.; Bai, J. F.; Zheng, B. S.; Li, Y. Z.; Li, S. H. Highly selective CO2 capture of an agw-type metal-organic framework with inserted amides: Experimental and theoretical studies. Chem. Commun.2012, 48, 3058–3060.
Stoeck, U.; Senkovska, I.; Bon, V.; Krause, S.; Kaskel, S. Assembly of metal-organic polyhedra into highly porous frameworks for ethene delivery. Chem. Commun.2015, 51, 1046–1049.
Guillerm, V.; Weseliiíski, L. J.; Belmabkhout, Y.; Cairns, A. J.; D’Elia, V.; Wojtas, L.; Adil, K.; Eddaoudi, M. Discovery and introduction of a (3,18)-connected net as an ideal blueprint for the design of metal-organic frameworks. Nat. Chem.2014, 6, 673–680.
Li, M.; Li, D.; O’Keeffe, M.; Yaghi, O. M. Topological Analysis of metal-organic frameworks with polytopic linkers and/or multiple building units and the minimal transitivity principle. Chem. Rev.2014, 114, 1343–1370.
Spek, A. L. PLATON, A Multipurpose Crystallographic Tool; Utrecht University: Utrecht, 2003.
Zheng, B. S.; Bai, J. F.; Duan, J. G.; Wojtas, L.; Zaworotko, M. J. Enhanced CO2 binding affinity of a high-uptake rht-type metal-organic framework decorated with acylamide groups. J. Am. Chem. Soc.2011, 133, 748–751.
Li, H.; Li, L. B.; Lin, R. B.; Ramirez, G.; Zhou, W.; Krishna, R.; Zhang, Z. J.; Xiang, S. C.; Chen, B. L. Microporous metal-organic framework with dual functionalities for efficient separation of acetylene from light hydrocarbon mixtures. ACS Sustainable Chem. Eng.2019, 7, 4897–4902.
Luo, F.; Yan, C. S.; Dang, L. L.; Krishna, R.; Zhou, W.; Wu, H.; Dong, X. L.; Han, Y; Hu, T. L.; O’Keeffe, M. et al. UTSA-74: A MOF-74 isomer with two accessible binding sites per metal center for highly selective gas separation. J. Am. Chem. Soc.2016, 138, 5678–5684.
Cui, X. L.; Chen, K. J.; Xing, H. B.; Yang, Q. W.; Krishna, R.; Bao, Z. B.; Wu, H.; Zhou, W.; Dong, X. L.; Han, Y. et al. Pore chemistry and size control in hybrid porous materials for acetylene capture from ethylene. Science2016, 353, 141–144.
Lee, J.; Chuah, C. Y.; Kim, J.; Kim, Y.; Ko, N.; Seo, Y.; Kim, K.; Bae, T. H.; Lee, E. Separation of acetylene from carbon dioxide and ethylene by a water-stable microporous metal-organic framework with aligned imidazolium groups inside the channels. Angew. Chem., Int. Ed.2018, 57, 7869–7873.
Scott, H. S.; Shivanna, M.; Bajpai, A.; Madden, D. G.; Chen, K. J.; Pham, T.; Forrest, K. A.; Hogan, A.; Space, B.; Perry, J. J. et al. Highly selective separation of C2H2 from CO2 by a new dichromate-based hybrid ultramicroporous material. ACS Appl. Mater. Interfaces2017, 9, 33395–33400.
Chen, K. J.; Scott, H. S.; Madden, D. G.; Pham, T.; Kumar, A.; Bajpai, A.; Lusi, M.; Forrest, K. A.; Space, B.; Perry IV, J. J. et al. Benchmark C2H2/CO2 and CO2/C2H2 separation by two closely related hybrid ultramicroporous materials. Chem2016, 1, 753–765.
Ye, Y. X.; Ma, Z. L.; Lin, R. B.; Krishna, R.; Zhou, W.; Lin, Q. J.; Zhang, Z. J.; Xiang, S. C.; Chen, B. L. Pore space partition within a metal-organic framework for highly efficient C2H2/CO2 separation. J. Am. Chem. Soc.2019, 141, 4130–4136.
Zeng, H.; Xie, M.; Huang, Y. L.; Zhao, Y. F.; Xie, X. J.; Bai, J. P.; Wan, M. Y.; Krishna, R.; Lu, W. G.; Li, D. Induced fit of C2H2 in a flexible MOF through cooperative action of open metal sites. Angew. Chem., Int. Ed.2019, 58, 8515–8519.
Peng, Y. L.; Pham, T.; Li, P. F.; Wang, T.; Chen, Y.; Chen, K. J.; Forrest, K. A.; Space, B.; Cheng, P.; Zaworotko, M. J. et al. Robust ultramicroporous metal-organic frameworks with benchmark affinity for acetylene. Angew. Chem., Int. Ed.2018, 57, 10971–10975.
Dong, Q. B.; Guo, Y. N.; Cao, H. F.; Wang, S. N.; Matsuda, R.; Duan, J. G. Accelerated C2H2/CO2 separation by a Se-functionalized porous coordination polymer with low binding energy. ACS Appl. Mater. Interfaces2020, 12, 3764–3772.
Zhou, B. H.; Zheng, J. J.; Duan, J. G.; Hou, C. C.; Wang, Y.; Jin, W. Q.; Xu, Q. Chemically robust, Cu-based porous coordination polymer nanosheets for efficient hydrogen evolution: Experimental and theoretical studies. ACS Appl. Mater. Interfaces2019, 11, 21086–21093.
Chen, Y.; Wang, B.; Wang, X. Q.; Xie, L. H.; Li, J. P.; Xie, Y. B.; Li, J. R. A copper(II)-paddlewheel metal-organic framework with exceptional hydrolytic stability and selective adsorption and detection ability of aniline in water. ACS Appl. Mater. Interfaces2017, 9, 27027–27035.
Duan, J. G.; Jin, W. Q.; Krishna, R. Natural gas purification using a porous coordination polymer with water and chemical stability. Inorg. Chem.2015, 54, 4279–4284.
Chai, J. D.; Head-Gordon, M. Long-range corrected hybrid density functionals with damped atom-atom dispersion corrections. Phys. Chem. Chem. Phys.2008, 10, 6615–6620.
Dolg, M.; Wedig, U.; Stoll, H.; Preuss, H. Energy-adjusted ab initio pseudopotentials for the first row transition elements. J. Chem. Phys.1987, 86, 866–872.
Boys, S. F.; Bernardi, F. The calculation of small molecular interactions by the differences of separate total energies. Some procedures with reduced errors. Mol. Phys.1970, 19, 553–566.
Frisch, M. J.; Trucks, G. W.; Schlegel, H. B.; Scuseria, G. E.; Robb, M. A.; Cheeseman, J. R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G. A. et. al. Gaussian 09, revision A.02; Gaussian, Inc.: Wallingford, CT, USA, 2009.
Nugent, P.; Belmabkhout, Y.; Burd, S. D.; Cairns, A. J.; Luebke, R.; Forrest, K.; Pham, T.; Ma, S. Q.; Space, B.; Wojtas, L. et al. Porous materials with optimal adsorption thermodynamics and kinetics for CO2 separation. Nature2013, 495, 80–84.
Acknowledgements
We thank the financial support of the National Natural Science Foundation of China (No. 21671102 and 21973029), the Innovative Research Team Program by the Ministry of Education of China (No. IRT-17R54), the Hunan Provincial Natural Science Foundation of China (No. 2020JJ4290), the Young and Middle-aged Academic Leader of Jiangsu Provincial Blue Project, the Six Talent Peaks Project in Jiangsu Province (No. JY-030) and the State Key Laboratory of Materials-Oriented Chemical Engineering (No. ZK201803). We also thank Prof. M. O’Keeffe and Prof. M. Li for valuable suggestion on topology analysis.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Chen, S., Behera, N., Yang, C. et al. A chemically stable nanoporous coordination polymer with fixed and free Cu2+ ions for boosted C2H2/CO2 separation. Nano Res. 14, 546–553 (2021). https://doi.org/10.1007/s12274-020-2935-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12274-020-2935-1