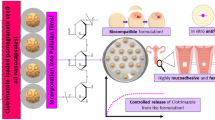
Abstract
Purpose
Infectious diseases caused by Candida sp. have high drug resistance due to the uncontrolled use of antibiotics. Among the natural products, ursolic acid (UA), a triterpene, has been increasing interest due to its antimicrobial effects. However, the physicochemical properties of UA, such as its low aqueous solubility, lead to failure of therapeutic application. Because of this, drug release systems, such as liquid crystals (LCs), can improve solubilization and increase the therapeutic efficacy of many drugs.
Methods
In this work, we evaluated the antifungal potential of UA and loaded into LC precursor system against Candida sp. The system was composed of oleic acid (30%), polysorbate 80 (60%), and purified water (10%). Polarized light microscopy, rheological assays, and simulated vaginal fluid (SVF) simulations were performed. Cytotoxicity assay was evaluated against L-929 cells (murine fibroblast), and antifungal evaluation was performed by microplate microdilution method against Candida albicans, Candida glabrata, Candida krusei, Candida parapsilosis, and Candida tropicalis.
Results
The system showed acceptable parameters for UA incorporation, such as adhesiveness, texture, and flow behaviors, compatible to vaginal administration. Free UA showed no antifungal activity; however, after incorporation into LCs, it was observed against C. albicans, C. glabrata, C. tropicalis, and C. parapsilosis (MIC values around 500 μg/mL and 3.9 μg/cell viability assays showed moderate cytotoxicity [halo diameter 1.00 ± 0.156]).
Conclusion
In conclusion, the results corroborate in the materials field as a possible alternative in the treatment of infectious diseases by Candida species and the systems showed potential promising for enhancing AU antifungal performance with the application on vulvovaginitis.



Similar content being viewed by others
Change history
28 August 2020
A Correction to this paper has been published: https://doi.org/10.1007/s12247-020-09489-3
Abbreviations
- AmB:
-
Amphotericin B
- DMEM:
-
Culture Medium Dulbecco
- F:
-
Formulation containing 60% (w/w) of surfactant, 30% (w/w) of oleic acid, and 10% (w/w) of purified water
- F100:
-
Formulation containing 60% (w/w) of surfactant, 30% (w/w) of oleic acid, and 10% (w/w) of purified water diluted with 100% (v/w) of simulated vaginal fluid
- F30:
-
Formulation containing 60% (w/w) of surfactant, 30% (w/w) of oleic acid, and 10% (w/w) of purified water diluted with 30% (v/w) of simulated vaginal fluid
- F50:
-
Formulation containing 60% (w/w) of surfactant, 30% (w/w) of oleic acid, and 10% (w/w) of purified water diluted with 50% (v/w) of simulated vaginal fluid
- FLC:
-
Fluconazole
- FUA:
-
Formulation containing 60% (w/w) of surfactant, 30% (w/w) of oleic acid, and 10% (w/w) of purified water loaded with ursolic acid (2000 μg/mL)
- FUA100:
-
Formulation containing 60% (w/w) of surfactant, 30% (w/w) of oleic acid, and 10% (w/w) of purified water loaded with ursolic acid (2000 μg/mL) diluted with 100% (v/w) of simulated vaginal fluid
- FUA30:
-
Formulation containing 60% (w/w) of surfactant, 30% (w/w) of oleic acid, and 10% (w/w) of purified water loaded with ursolic acid (2000 μg/mL) diluted with 30% (v/w) of simulated vaginal fluid
- FUA50:
-
Formulation containing 60% (w/w) of surfactant, 30% (w/w) of oleic acid, and 10% (w/w) of purified water loaded with ursolic acid (2000 μg/mL) diluted with 50% (v/w) of simulated vaginal fluid
- GTS:
-
Gelled transparent systems
- LCs:
-
Liquid crystals
- LTS:
-
Liquid transparent systems
- MEs:
-
Microemulsions
- MFC:
-
Minimum fungicidal concentration
- MIC:
-
Minimum inhibitory concentration
- NC:
-
Negative control
- OA:
-
Oleic acid
- PC:
-
Positive control
- PS:
-
Phase separation
- RPMI:
-
Roswell Park Memorial Institute—1640 medium
- SDB:
-
Sabouraud dextrose broth
- SVF:
-
Simulated vaginal fluid
- TTC:
-
Triphenyltetrazolium chloride
- UA:
-
Ursolic acid
References
Ramos MAS, Silva PB, Spósito L, Toledo LG, Bonifácio BV, Rodero CF, et al. Nanotechnology-based drug delivery systems for control of microbial biofilms: a review. Int J Nanomedicine. 2018;13:1179–213.
Fisher MC, Hawkins NJ, Sanglard D, Gurr SJ. Health and food security—628 TCLocal. Science. 80(742):739–42.
Cortegiani A, Misseri G, Fasciana T, Giammanco A, Giarratano A, Chowdhary A. Epidemiology, clinical characteristics, resistance, and treatment of infections by Candida auris. J Intensive Care. 2018;6(1):1–13.
Pappas GP, Lionakis MS, Arendrup MC, Zeichner LO, Kullberg BJ. Invasive candidiasis. Infect Dis Clin N Am. 2018;30(1):103–24.
Mellinghoff SC, Von Bergwelt-Baildon M, Schoßer HA, Cornely OA. A novel approach to candidemia? The potential role of checkpoint inhibition. Med Mycol. 2019;57(2):151–4.
Chen H, Gao Y, Wang A, Zhou X, Zheng Y, Zhou J. Evolution in medicinal chemistry of ursolic acid derivatives as anticancer agents. Eur J Med Chem. 2015;92:648–55.
Jin YR, Jin JL, Li CH, Piao XX, Jin NG. Ursolic acid enhances mouse liver regeneration after partial hepatectomy. Pharm Biol. 2012;50(4):523–8.
Saravanan R, Viswanathan P, Pugalendi KV. Protective effect of ursolic acid on ethanol-mediated experimental liver damage in rats. Life Sci. 2006;78(7):713–8.
Nascimento PGG, Lemos TLG, Bizerra AMC, Arriaga AMC, Ferreira DA, Santiago GMP, et al. Antibacterial and antioxidant activities of ursolic acid and derivatives. Molecules. 2014;19(1):1317–27.
Kurek A, Nadkowska P, Pliszka S, Wolska KI. Modulation of antibiotic resistance in bacterial pathogens by oleanolic acid and ursolic acid. Phytomedicine. 2012;19(6):515–9.
Bonifácio BV, Silva PB, Ramos MAS, Negri MSK, Bauab TM, Chorilli M. Nanotechnology-based drug delivery systems and herbal medicines: a review. Int J Nanomedicine. 2013;9(1):1–15.
Jiang Z, Kempinski C, Chappell J. Extraction and analysis of terpenes/terpenoids. Curr Protoc Plant Biol. 2016;1(2):345–58.
Ghosh V, Saranya S, Mukherjee A, Chandrasekaran N. Antibacterial microemulsion prevents sepsis and triggers healing of wound in Wistar rats. Colloids Surf B: Biointerfaces. 2013;105:152–7.
Rajendran R, Radhai R, Kotresh TM, Csiszar E. Development of antimicrobial cotton fabrics using herb loaded nanoparticles. Carbohydr Polym. 2013;91(2):613–7.
Salmazi R, Calixto G, Bernegossi J, Ramos MAS, Bauab TM, Chorilli M. A curcumin-loaded liquid crystal precursor mucoadhesive system for the treatment of vaginal candidiasis. Int J Nanomedicine. 2015;10:4815–24.
Li W, Zhou J, Xu Y. Study of the in vitro cytotoxicity testing of medical devices. Biomed Rep. 2015;3:617–20.
CLSI. M27-A3: reference method for broth dilution antifungal susceptibility testing of yeasts. Wayne: Clinical Laboratory Standards Institute; 2008.
Miyashiro CA, Bernegossi J, Bonifácio BV, Toledo LG, Ramos MAS, Bauab TM, et al. Development and characterization of a novel liquid crystalline system containing sodium alginate for incorporation of trans-resveratrol intended for treatment of buccal candidiasis. Pharmazie. 2020;1(75):178–84.
Ceulemans J, Vinckier I, Ludwig A. The use of xanthan gum in an ophthalmic liquid dosage form: rheological characterization of the interaction with mucin. J Pharm Sci. 2002;91:1117–27.
Carvalho FC, Calixto G, Hatakeyama IN, Luz GM, Gremião MPD, Chorilli M. Rheological, mechanical, and bioadhesive behavior of hydrogels to optimize skin delivery systems. Drug Dev Ind Pharm. 2013;39:1750–7.
Calixto GMF, Garcia MH, Cilli EM, Chiavacci LA, Chorilli M. Design and characterization of a novel p1025 peptide-loaded liquid crystalline system for the treatment of dental caries. Molecules. 2016;21:158.
Fonseca-Santos B, Bonifácio BV, Baub TM, Gremião MPD, Chorilli M. In-situ gelling liquid crystal mucoadhesive vehicle for curcumin buccal administration and its potential application in the treatment of oral candidiasis. J Biomed Nanotechnol. 2019;15:1334–44.
Fonseca-Santos B, Ramos MA, Rodero CF, Gremião MPD, Chorilli M. Design, characterization, and biological evaluation of curcumin-loaded surfactant-based systems for topical drug delivery. Int J Nanomedicine. 2016;11:4553–62.
Fonseca-Santos B, Satake CY, Calixto GMF, Ramos MAS, Chorilli M. Trans-resveratrol-loaded nonionic lamellar liquid-crystalline systems: structural, rheological, mechanical, textural, and bioadhesive characterization and evaluation of in vivo anti-inflammatory activity. Int J Nanomedicine. 2017;12:6883–93.
Calixto GMF, Victorelli FD, Dovigo LN, Chorilli M. Polyethyleneimine and chitosan polymer-based mucoadhesive liquid crystalline systems intended for buccal drug delivery. AAPS PharmSciTech. 2018;19:820–36.
Carvalho FC, Barbi MS, Sarmento VHV, Chiavacci LA, Netto FM, Gremião MPD. Surfactant systems for nasal zidovudine delivery: structural, rheological and mucoadhesive properties. J Pharm Pharmacol. 2010;62:430–9.
Araújo PR, Calixto GMF, Silva IC, Zago PLH, Oshiro Junior JA, Pavan FR, et al. Mucoadhesive in situ gelling liquid crystalline precursor system to improve the vaginal administration of drugs. AAPS PharmSciTech. 2019;20:225–37.
Deshkar SS, Palve VK. Formulation and development of thermosensitive cyclodextrin-based in situ gel of voriconazole for vaginal delivery. J Drug Deliv Sci Technol. 2019;49(November 2018):277–85.
Rençber S, Karavana SY, Şenyiğit ZA, Eraç B, Limoncu MH, Baloğlu E. Mucoadhesive in situ gel formulation for vaginal delivery of clotrimazole: formulation, preparation, and in vitro/in vivo evaluation. Pharm Dev Technol. 2017;22(4):551–61.
Bernegossi J, Calixto GMF, Silva Sanches PR, Fontana CR, Cilli EM, Garrido SS, et al. Peptide KSL-W-loaded mucoadhesive liquid crystalline vehicle as an alternative treatment for multispecies oral biofilm. Molecules. 2016;21:1–14.
Bruschi ML. Strategies to modify the drug release from pharmaceutical systems; 2015. p. 1–199.
Formariz TP, Cocenza Urban MC, Da Silva AA, Gremião MPD, De Oliveira AG. Microemulsões e fases líquidas cristalinas como sistemas de liberação de fármacos. Rev Bras Ciencias Farm J Pharm Sci. 2005;41(3):301–13.
Goodby JWG, Spiess HW, Vill V. Handbook of liquid crystals. 2rd ed. Berlin: Wiley VCH; 1998.
Fehér A, Csányi E, Erös I. In situ forming lyotropic liquid crystalline systems containing metronidazole-benzoate. J Drug Deliv Sci Technol. 2005;15(5):343–6.
Malmsten M. Phase transformations in self-assembly systems for drug delivery applications. J Dispers Sci Technol. 2007:63–72.
Malmsten M. Surfactants and Polymers in Drug Delivery. J Control Release. 2003:415–416.
Garti N, Mezzenga R, Somasundaran P. Self-assembled supramolecular architectures: lyotropic liquid crystals; 2012. p. 396.
Jones DS, Woolfson AD, Brown AF. Textural, viscoelastic and mucoadhesive properties of pharmaceutical gels composed of cellulose polymers. Int J Pharm. 1997;151:223–33.
Jones DS, Woolfson AD, Brown AF, Coulter WA, McClelland C, Irwin CR. Design, characterisation and preliminary clinical evaluation of a novel mucoadhesive topical formulation containing tetracycline for the treatment of periodontal disease. J Control Release. 2000;67:357–68.
Karavasili C, Fatouros DG. Smart materials: in situ gel-forming systems for nasal delivery. Drug Discov Today. 2016:157–60.
Rupenthal ID, Green CR, Alany RG. Comparison of ion-activated in situ gelling systems for ocular drug delivery. Part 1: physicochemical characterisation and in vitro release. Int J Pharm. 2011;411:69–77.
Moreno MA, Frutos P, Ballesteros MP. Lyophilized lecithin based oil-water microemulsions as a new and low toxic delivery system for amphotericin B. Pharm Res. 2001;18:344–51.
Kantarci G, Özgüney I, Karasulu HY, Arzik S, Güneri T. Comparison of different water/oil microemulsions containing diclofenac sodium: preparation, characterization, release rate, and skin irritation studies. AAPS PharmSciTech. 2007;8(4):1–7.
Lee CH, Moturi V, Lee Y. Thixotropic property in pharmaceutical formulations. J Control Release. 2009:88–98.
Chorilli M, Prestes PS, Rigon RB, Leonardi GR, Chiavacci LA, Sarmento VHV, et al. Structural characterization and in vivo evaluation of retinyl palmitate in non-ionic lamellar liquid crystalline system. Colloids Surf B: Biointerfaces. 2011;85:182–8.
Cordobés F, Franco JM, Gallegos C. Rheology of the lamellar liquid-crystalline phase in polyethoxylated alcohol/water/heptane systems. Grasas Aceites. 2005;56:96–105.
Montalvo G, Valiente M, Rodenas E. Rheological properties of the L phase and the hexagonal, lamellar, and cubic liquid crystals of the CTAB/benzyl alcohol/water system. Langmuir. 1996;12:3202–8.
Nixon JR, Chawla BPS. Solubilization and rheology of the system ascorbic acid-water-polysorbate 80: temperature effects. J Pharm Pharmacol. 1969;21:79–84.
Richtering W. Fundamentals of interface and colloid science: volume IV: particulate colloids and volume V: soft colloids. Appl Rheol. 2019;15(5):310.
Alsarra IA, Hamed AY, Alanazi FK, Neau SH. Rheological and mucoadhesive characterization of poly(vinylpyrrolidone) hydrogels designed for nasal mucosal drug delivery. Arch Pharm Res. 2011;34:573–82.
Alsarra IA, Hamed AY, Mahrous GM, El Maghraby GM, Al-Robayan AA, Alanazi FK. Mucoadhesive polymeric hydrogels for nasal delivery of acyclovir polymeric hydrogels for nasal delivery of acyclovir. Drug Dev Ind Pharm. 2009;35:352–62.
Carvalho FC, Campos ML, Peccinini RG, Gremião MPD. Nasal administration of liquid crystal precursor mucoadhesive vehicle as an alternative antiretroviral therapy. Eur J Pharm Biopharm. 2013;84:219–27.
Arancibia C, Bayarri S, Costell E. Effect of hydrocolloid on rheology and microstructure of high-protein soy desserts. J Food Sci Technol. 2015;52:6435–44.
Cardoso VMO, Cury BSF, Evangelista RC, Gremião MPD. Development and characterization of cross-linked gellan gum and retrograded starch blend hydrogels for drug delivery applications. J Mech Behav Biomed Mater. 2017;65:317–33.
Rattan S, Li L, Lau HK, Crosby AJ, Kiick KL. Micromechanical characterization of soft, biopolymeric hydrogels: stiffness, resilience, and failure. Soft Matter. 2018;14:3478–89.
Saxena A, Kaloti M, Bohidar HB. Rheological properties of binary and ternary protein-polysaccharide co-hydrogels and comparative release kinetics of salbutamol sulphate from their matrices. Int J Biol Macromol. 2011;48:263–70.
Rodero CF, Calixto GMF, Santos KC, Sato MR, Ramos MAS, Miró MS, et al. Curcumin-loaded liquid crystalline systems for controlled drug release and improved treatment of vulvovaginal candidiasis. Mol Pharm. 2018;15:4491–504.
Rigon RB, Fachinetti N, Severino P, Santana MHA, Chorilli M. Skin delivery and in vitro biological evaluation of trans-resveratrol-loaded solid lipid nanoparticles for skin disorder therapies. Molecules. 2016;21:116–29.
Fachinetti N, Rigon RB, Eloy JO, Sato MR, Santos KC, Chorilli M. Comparative study of glyceryl behenate or polyoxyethylene 40 stearate-based lipid carriers for trans-resveratrol delivery: development, characterization and evaluation of the in vitro tyrosinase inhibition. AAPS PharmSciTech. 2018;19:1401–9.
Jabeen K, Javaid A, Ahmad E, Athar M. Antifungal compounds from Melia azedarach leaves for management of Ascochyta rabiei, the cause of chickpea blight. Nat Prod Res. 2011;25(3):264–76.
Bitencourt FG, D Vieira PB, Meirelles LC, Rigo GV, Silva EF, SCB G, et al. Anti-Trichomonas vaginalis activity of ursolic acid derivative: a promising alternative. Parasitol Res. 2018;117:1573–80.
Ramos MAS, Silva PB, Toledo LG, Oda FB, Silva IC, Santos LC, et al. Intravaginal delivery of Syngonanthus nitens (Bong.) Ruhland fraction based on a nanoemulsion system applied to vulvovaginal candidiasis treatment. J Biomed Nanotechnol. 2019;15:1072–89.
Ramos MAS, Toledo LG, Calixto GMF, Bonifácio BV, Araújo MGF, Santos LC, et al. Syngonanthus nitens Bong. (Rhul.)-loaded nanostructured system for vulvovaginal candidiasis treatment. Int J Mol Sci. 2016;17(8):1368–87.
Ramos MAS, Calixto G, Toledo LG, Bonifácio BV, Santos LC, Almeida MTG, et al. Liquid crystal precursor mucoadhesive system as a strategy to improve the prophylactic action of Syngonanthus nitens (Bong.) Ruhland against infection by Candida krusei. Int J Nanomedicine. 2015;10:7455–66.
Victorelli FD, Calixto GMF, Ramos MAS, Bauab TM, Chorilli M. Metronidazole-loaded polyethyleneimine and chitosan-based liquid crystalline system for treatment of staphylococcal skin infections. J Biomed Nanotechnol. 2018;15:1072–89.
Bernegossi J, Fontana AR, Caiaffa KS, Duque C, Chorilli M. Inhibitory effect of a KSL-W peptide-loaded poloxamer 407-based microemulsions for buccal delivery on Fusobacterium nucleatum biofilm. J Biomed Nanotechnol. 2020;16:390–7.
Mayer FL, Wilson D, Hube B. Candida albicans pathogenicity mechanisms. Virulence. 2013:119–28.
Silva S, Negri M, Henriques M, Oliveira R, Williams DW, Azeredo J. Candida glabrata, Candida parapsilosis and Candida tropicalis: biology, epidemiology, pathogenicity and antifungal resistance. FEMS Microbiol Rev. 2012;36:288–305.
Acknowledgments
We thank the São Paulo Research Foundation—FAPESP (grant #2018/23442-2) for the financial support. This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Ensino Superior—Brasil (CAPES)—Finance code 001.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Marena, G.D., Fonseca-Santos, B., Matheus Aparecido dos Santos, R. et al. Incorporation of Ursolic Acid in Liquid Crystalline Systems Improves the Antifungal Activity Against Candida Sp. J Pharm Innov 16, 576–586 (2021). https://doi.org/10.1007/s12247-020-09470-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12247-020-09470-0