Abstract
One-compartment hydrogen peroxide fuel cells with Co, Cu, and Fe phthalocyanine (PC) and iron nitride (FexN) as cathodes and Ni anode have been investigated as sustainable energy sources. The cells were operated under acidic conditions and at room temperature. The potentials for onset of the catalytic currents in these cells were determined via cyclic voltammograms. The reduction current onset potentials of FePC, CoPC, CuPC and FexN were 0.56 V, 0.42 V, 0.51 V and 0.57 V, respectively. Potential-current linear sweep voltammetry was utilized to determine the open circuit potentials (OCP) and the power densities the fuel cells. The OCPs for Co, Cu, and Fe phthalocyanines and FexN were 0.47 V, 0.57 V, 0.56 V and 0.58 V, respectively. The maximum output power densities of FePC and CoPC, CuPC, and FexN were 3.41 mW cm−2, 0.39 mW cm−2, 0.39 mW cm−2 and 0.76 mW cm−2, respectively. These power densities are suitable for powering micro-devices.
Export citation and abstract BibTeX RIS

This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 License (CC BY, http://creativecommons.org/licenses/by/4.0/), which permits unrestricted reuse of the work in any medium, provided the original work is properly cited.
In order to address the challenge of climate change, environmental-friendly energy producing technologies are gaining attention. Besides wind and solar power, fuel cells offer an alternative that can potentially utilize abundant natural resources. To address development of fuel cells that utilize green processes requires the investigation of environmentally friendly catalysts, in combination with clean reagents, such as oxygen and hydrogen peroxide for oxidation reactions. We describe below our results of hydrogen peroxide based fuel cells that are not only carbon- free but can also serve both as fuel (electron donor) and as an oxidant (electron acceptor) in a single chamber fuel cell without a separator membrane. Moreover, H2O2 has high energy density, is cheap, clean, safe, and produces water as a by-product. Another advantage is that solar energy can be used to produce H2O2 photo-catalytically under acidic conditions from H2O, which can then be stored for later generation of electricity in a fuel cell.1
Metallophthalocyanine catalysts
In H2O2 fuel cells, oxygen reduction reaction (ORR) at the cathode is a half reaction that generally determines the performance of these devices. Hence, choice of an appropriate electrocatalyst as the cathode can enhance the ORR. Although Pt would serve this purpose well, its prohibitive cost has prompted search for alternatives. In nature, the most common enzymes found in plants and animals contain porphyrin complexes, which are responsible for catalytic aerobic oxidation, reduction, transport of dioxygen, as well as destruction of peroxides.2
Metallophthalocyanines (MPCs) are structurally related metal complexes to porphyrins and can be produced synthetically.3 In such metal-nitrogen–carbon (M–N–C) based catalysts, incorporation of metal atoms into the phthalocyanine (PC) ring produces a π-electron conjugation system in the PC molecular structure that results in high oxidation and reduction properties.4–7 MPCs are attractive catalysts since they are inexpensive and available in a large scale since they are used in several industrial applications.3 Their thermal and environmental stability are important considerations for their applications in fuel cells. In what follows below, we have examined and compared single chamber H2O2 fuel cells with Fe, Cu, and Co transition metal phthalocyanines as the catalysts operating at room temperature.
The choice of these 3 phthalocyanines is based on their catalytic properties. In nature, Cu based complexes appear in many forms of life, ranging from bacteria to humans, as catalysts for oxygen reduction to water.8,9 Also, CoPC has been shown to be a highly effective electrocatalyst and has recently been employed as an effective catalyst in microbial fuel cells.10–13 Iron–nitrogen–carbon (Fe–N–Cs) containing materials, such as FePC, are some of the most effective catalysts for ORRs where the FeN4 moieties serve as active sites.14,15 This has been attributed to the uncommon spin state of a Fe(II) electron that enhances the ORR process.16,17 In the M–N–C materials, the metal centers are bound to the nitrogen atoms in the complex that act as active sites, whereas the carbonaceous support is believed to improve the electron transfer.18 Hence, a fourth catalyst, FexN (x = 2–4) was also examined, to compare its fuel cell performance with FePC. Cu, Co and Fe phthalocyanines were purchased from Sigma-Aldrich and FexN from Beantown Chemical.
H2O2 fuel cells
H2O2 fuel cells have been demonstrated in acidic-basic electrolytes, separated with a Nafion membrane.19 Separation of anode and cathode, each at different pH results in enhancement in the output power but makes the system more expensive and complex.7 Cells based on a microfluidic channel have also been reported where mixing is confined at a specific location to achieve liquid-liquid interface.20 However, unlike hydrogen fuel cells, H2O2 can act as both an oxidant and a reductant. Then by selecting appropriate anode and cathode materials, based on their catalytic activity, the cell configuration can be simplified into a single chamber, membraneless cell.21 The reason for choosing MPCs for this investigation is that these complexes can mediate selective oxidation reactions dependent on the choice of the oxidant in a single chamber reactor.22 This selectivity is important because although catalysts such as Pt can provide efficient reduction of H2O2 to water, it can also cause its direct decomposition to oxygen.23 Hence, in a single cell configuration, electrodes with different reactivity to oxidation and reduction of H2O2 can be employed to facilitate the following reactions21:
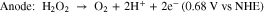
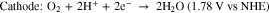
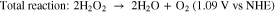
The theoretical electromotive force for these reactions is 1.09 V which is comparable to the theoretical open-circuit voltage (OCV) of a hydrogen–oxygen fuel cell (1.23 V).21 The final products in this case are water and oxygen.
Single compartment hydrogen peroxide cells operating under either alkaline and acidic environments have been reported where the catalysts have included metal (Pt, Pd, Ni and Au) wires, Pb-Ag nanoparticles, ferric ferrocyanide (Prussian Blue), and protonated iron phthalocyanine [FeIII(Pc)Cl].7,21,24,25 In this investigation, we have compared performance of iron, cobalt, and copper phthalocyanines and FexN as cathodes, in single chamber, acidic H2O2 fuels cells. The anode consisted of a strip of a nickel mesh.
Materials and Methods
The electrocatalytic reduction of H2O2 was examined in a three-electrode cell with a strip of carbon paper coated with MPC or FexN as cathodes, a Ni mesh as the anode, and an Ag/AgCl reference electrode. The Ni anode and MPC cathode act as selective electrodes for the H2O2 oxidation and reduction, respectively. Thus, a one-compartment fuel cell without a membrane separator between the anode and cathode can be realized. One of the drawbacks of PC complexes is their low electron conductivity.6,26 Hence, to improve the electrocatalytic activity, conductive materials were necessary in preparing the electrodes. For this purpose, the MPCs were mixed with carbon black, multi walled carbon nanotubes, and polyvinylidene difluoride (PVDF) as the binder. The ratios of the powder mixtures were 80% MPC powder, 9% MWCNTs, 9% PVDF binder, and 2% carbon black. N-Methyl-2-pyrrolidone (NMP) was added to the powder mixtures to produce a paste which was applied to carbon paper strips that had been rinsed with ethanol and dried in an oven at 60 °C for 15 min. The physical surface area of the applied region was 1 cm2. The electrodes were left to dry overnight. Lastly, the electrodes were coated with 0.2% wt. Nafion and annealed at 110 °C under low vacuum for 1 h and cooled overnight.
Results and Discussion
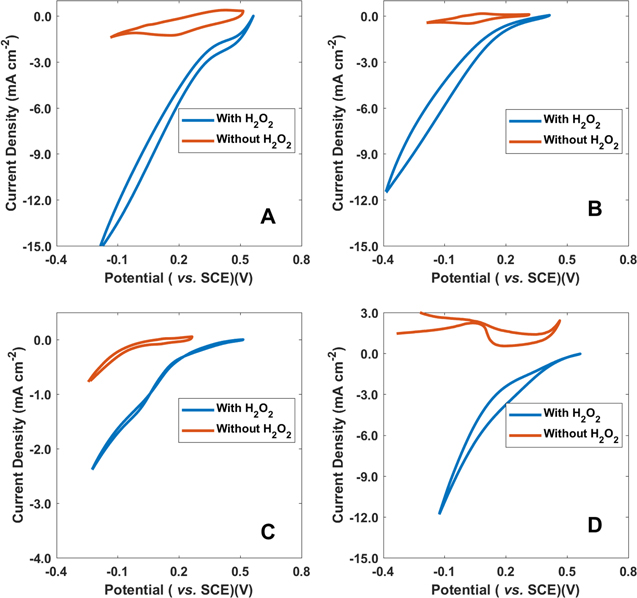
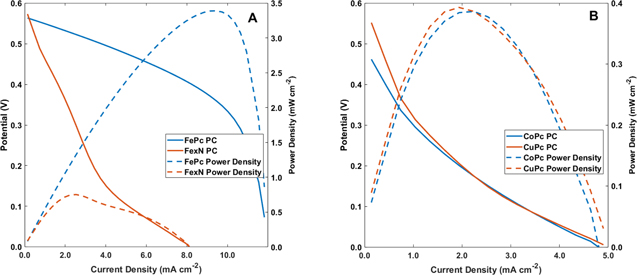
Cyclic voltammetry experiments were carried out with the electrodes immersed in an electrolyte that consisted of 40 ml equivolume of 0.5 M H2O2 and 0.1 M HCl. Ag/AgCl served as the reference electrode (reported vs SCE) and 1 cm diameter carbon rods were used as the counter electrodes for the experiments. A Gamry potentiostat was used for the electrocatalytic measurements. Before the experiments began, the cells were allowed to stabilize for 10 min. All experiments were done at 10 mV s−1 sweep rate. Each material was examined in electrolytes with and without H2O2 to determine the background effect. Cyclic voltammograms of the 4 catalysts examined are shown in Fig. 1, where the voltage sweeps without H2O2 are shown in orange and with H2O2 in blue. From the sweeps with H2O2, the potentials for onset of the catalytic currents of the peroxide reduction were observed. The reduction onset potentials of FePC, CoPC, CuPC and FexN were 0.56 V, 0.42 V, 0.51 V and 0.57 V, respectively. The performances of the fuel cells were examined by recording polarization curves via linear potential sweep voltammetry to determine the potential–current and power density characteristics. The sweep rate for the polarization curves was 1 μA cm−2 and the electrolyte composition was the same as for voltammetry. The results are shown in Fig. 2. Open circuit potentials (OCPs) were measured at the beginning of the polarization curves. The OCPs for FePC, CoPc, CuPc, and FexN were determined to be 0.56 V, 0.47 V, 0.57 V, and 0.58 V, respectively. The maximum output power densities of FePC and CoPC, CuPC, and FexN were 3.41 mW cm−2, 0.39 mW cm−2, 0.39 mW cm−2 and 0.76 mW cm−2, respectively. These results are summarized in Table I. Clearly, the power density of FePC is more than an order of magnitude higher than of CoPC and CuPC. The power density of FePC is also significantly higher than the reported for protonated iron phthalocyanine cathode in membraneless hydrogen peroxide fuel cells and Ni anodes.21
Figure 1. Cyclic voltammograms of H2O2 on carbon paper modified with supported M-PC complexes and FexN. (A) FePC, (B) CoPC, (C) CuPC and (D) FexN. The measurements were made in 0.1 M HCl with (in blue) and without 0.5 M H2O2 (orange). Scan rate of 10 mV s−1 was used for the experiments.
Download figure:
Standard image High-resolution imageFigure 2. Polarization curves of nickel mesh anode vs FePc and FeN (A), and CuPc and CoPc (B) cathodes in 40 ml equivolume of 0.5 M H2O2 and 0.1 M HCl. The scan rate used for the experiments was 100 μA s−1.
Download figure:
Standard image High-resolution imageTable I. Summary of the fuel cell parameters.
| Onset potential of H2O2 reduction (V) | OPC with Ni-mesh (V) | Power density with Ni-mesh (mW cm−2) | |
|---|---|---|---|
| CoPC | 0.42 | 0.47 | 0.39 |
| CuPC | 0.51 | 0.57 | 0.40 |
| FePC | 0.56 | 0.56 | 3.41 |
| FexN | 0.57 | 0.58 | 0.76 |
Although the active center of FePC is similar to the catalyst FexN, FePC produces a higher power density. This could be due to the presence of the carbonaceous support providing improved electron transfer as stated before.18
Conclusions
Membraneless, one-compartment H2O2 fuel cells have been investigated as potentially low cost, environmentally friendly energy sources. The catalysts on the cathodes were Cu, Co, and Fe phthalocyanines and FexN. Polarization measurements were performed to determine OCP and maximum power density. Of all the 3 MPC catalysts examined, CoPC had the lowest potential for the onset of reduction current, whereas FexN had the highest OCP, and FePC produced the highest power density. Considering all of these parameters, FePC showed the best performance for the H2O2 fuel cell operating at room temperature. Although further work is required for optimizing the performance of the membraneless fuel cell geometry, the power density it can produce could be sufficient for operating micro-devices. The single compartment structure and use of environmentally friendly H2O2 as the fuel and oxidizer demonstrates the potential of producing a sustainable energy source.