Endometrial and Menstrual Blood Mesenchymal Stem/Stromal Cells: Biological Properties and Clinical Application
- 1Reproductive Immunology Research Center, Avicenna Research Institute, Academic Center for Education, Culture and Research (ACECR), Tehran, Iran
- 2Oncopathology Research Center, Iran University of Medical Sciences, Tehran, Iran
- 3Centre for Reproductive Health, Hudson Institute of Medical Research, Melbourne, VIC, Australia
- 4The Ritchie Centre, Hudson Institute of Medical Research, Melbourne, VIC, Australia
- 5Department of Obstetrics and Gynaecology, Monash University, Melbourne, VIC, Australia
- 6Immunology Research Center, Iran University of Medical Sciences, Tehran, Iran
- 7Nanobitechnology Research Center, Avicenna Research Institute, Academic Center for Education, Culture and Research (ACECR), Tehran, Iran
- 8Department of Immunology, School of Public Health, Tehran University of Medical Sciences, Tehran, Iran
A highly proliferative mesenchymal stem/stromal cell (MSC) population was recently discovered in the dynamic, cyclically regenerating human endometrium as clonogenic stromal cells that fulfilled the International Society for Cellular Therapy (ISCT) criteria. Specific surface markers enriching for clonogenic endometrial MSC (eMSC), CD140b and CD146 co-expression, and the single marker SUSD2, showed their perivascular identity in the endometrium, including the layer which sheds during menstruation. Indeed, cells with MSC properties have been identified in menstrual fluid and commonly termed menstrual blood stem/stromal cells (MenSC). MenSC are generally retrieved from menstrual fluid as plastic adherent cells, similar to bone marrow MSC (bmMSC). While eMSC and MenSC share several biological features with bmMSC, they also show some differences in immunophenotype, proliferation and differentiation capacities. Here we review the phenotype and functions of eMSC and MenSC, with a focus on recent studies. Similar to other MSC, eMSC and MenSC exert immunomodulatory and anti-inflammatory impacts on key cells of the innate and adaptive immune system. These include macrophages, T cells and NK cells, both in vitro and in small and large animal models. These properties suggest eMSC and MenSC as additional sources of MSC for cell therapies in regenerative medicine as well as immune-mediated disorders and inflammatory diseases. Their easy acquisition via an office-based biopsy or collected from menstrual effluent makes eMSC and MenSC attractive sources of MSC for clinical applications. In preparation for clinical translation, a serum-free culture protocol was established for eMSC which includes a small molecule TGFβ receptor inhibitor that prevents spontaneous differentiation, apoptosis, senescence, maintains the clonogenic SUSD2+ population and enhances their potency, suggesting potential for cell-therapies and regenerative medicine. However, standardization of MenSC isolation protocols and culture conditions are major issues requiring further research to maximize their potential for clinical application. Future research will also address crucial safety aspects of eMSC and MenSC to ensure these protocols produce cell products free from tumorigenicity and toxicity. Although a wealth of data on the biological properties of eMSC and MenSC has recently been published, it will be important to address their mechanism of action in preclinical models of human disease.
Introduction
Almost all human tissues contain a small resident population of perivascular mesenchymal stem/stromal cells (MSC) (Crisan et al., 2008). MSC have also been identified in a wide variety of small and large mammalian species (Rozemuller et al., 2010), although these have been studied to a lesser degree. Here we discuss a novel source of MSC from the highly regenerative endometrial lining of the uterus (Figure 1A), accessible by biopsy in an office-based procedure without an anesthetic, or non-invasively from menstrual blood (Figure 1B) (Ulrich et al., 2013). This review will focus on the biological properties and recent functional characterization of endometrial MSC (eMSC) and menstrual blood MSC (MenSC). The reader is referred to a recent comprehensive review providing greater details on earlier studies describing the identification and early characterization of endometrial MSC, MenSC and endometrial stromal fibroblasts (Gargett et al., 2016). Both epithelial progenitors and MSC have been identified in human and mouse endometrium, however, it is the endometrial and menstrual blood MSC that comprise the topic of this review.

Figure 1. Ovarian and Menstrual cycle, structure of human endometrium and its shedding during menstruation. (A) Schematic showing human ovarian hormonal changes corresponding with endometrial growth, differentiation and shedding, during the menstruation, proliferative and secretory phases, respectively, of a menstrual cycle. (B) Schematic showing endometrial tissue collection from human endometrium as an office-based procedure using a Pipelle endometrial suction catheter and menstrual fluid using a menstrual cup. (C) Histological sections stained with H&E of pre-menopausal human endometrium during the growth (proliferative), differentiation (secretory) and menses stages of the menstrual cycle showing the functionalis, basalis and myometrial layers of the endometrium. *Endometrial tissue breakdown early in the menstrual stage, leaving behind an intact basalis layer. Menstrual blood showing endometrial tissue fragments comprising endometrial stroma, glands and blood cells. Dotted lines define the layers of the endometrium. g, glands. Reproduced with permission from Figure 1A from Elsevier (Gargett et al., 2008) and Figure 1C from Oxford University Press (Nguyen et al., 2012).
Recently the MSC field has been criticized, due to the poor characterization of MSC from various sources, which has resulted in underwhelming outcomes of many clinical trials using MSC (Sipp et al., 2018). Counter arguments reiterate the importance of using the appropriate definition of the cells under study (i.e., perivascular MSC versus fibroblasts) (Galipeau et al., 2019). Integrated transcriptomic profiling of human MSC from different tissues show their segregation by tissue of origin (Roson-Burgo et al., 2016; Menard et al., 2020) and distinct tissue-specific MSC immune signatures that are similar between fresh and cultured MSC from the same tissue source (Menard et al., 2020). Herein we will distinguish between perivascular eMSC and endometrial stromal fibroblasts. We will also differentiate between potential regenerative “stem cell” properties and immunomodulatory function of both eMSC and MenSC. It has become clear that the regenerative properties of MSC are mainly due to the trophic factors they secrete. These stimulate endogenous cells to repair damaged tissues, rather than MSC functioning as true stem cells (Bianco et al., 2013). MSC are reparative rather than regenerative. It is also recognized that MSC have profound immunomodulatory effects on innate and adaptive immune cells that promote healing by reducing inflammation and immune responses (Galipeau and Sensebe, 2018). In this review, we recognize these important developments in the MSC field and our review on endometrial and menstrual blood MSC and fibroblasts has been structured around these themes.
Human endometrium is a dynamic remodeling tissue, undergoing cycles of growth, differentiation and shedding on a monthly basis as part of the menstrual cycle (Gargett et al., 2012). These dynamic processes occur about 400 times in women until menopause (Jabbour et al., 2006). During menstruation, the upper functional layer of endometrial tissue sloughs off and the tissue fragments exit the body in menstrual blood, leaving a residual 1–2 mm of endometrial tissue (basalis) overlying the myometrium (uterine muscle) (Figures 1A–C). The raw surface rapidly reepithelializes and the new functional layer (functionalis), comprising epithelial-lined glands and an extensive vascularized stroma regenerates under the influence of rising, circulating estrogen levels (Gargett et al., 2008) in the next cycle. Atrophic postmenopausal endometrium, which transcriptionally resembles cycling basalis endometrium (Nguyen et al., 2012), also regenerates a functionalis-like layer when women take estrogen-only hormone replacement therapy. MSC can be harvested from this regenerated tissue (Ulrich et al., 2014b).
Human Endometrial MSC
Endometrial MSC were first identified as clonogenic stromal cells, comprising 1.3% of stromal fibroblasts harvested from hysterectomy tissue, which contains both functionalis and basalis endometrium (Chan et al., 2004). The large stromal colonies, comprised densely packed small cells with a fibroblast-like morphology and distinguishable from self-limiting small, sparsely packed colonies, likely originated from colony forming units-fibroblast (CFU-F) and do not vary in frequency during the menstrual cycle. This and their presence in postmenopausal endometrium indicate hormone independence of endometrial CFU-F (Schwab et al., 2005; Ulrich et al., 2014b). In comparison to single small colonies, individual large CFU-F showed high proliferative capacity, undergoing 30 population doublings (PD) and producing > 600 billion cells (Gargett et al., 2009). Single large endometrial CFU-F underwent self-renewal in vitro by serial cloning at very low seeding densities (5–10 cells/cm2) and differentiated into adipocytes, chondrocytes, myocytes and osteocytes (Gargett et al., 2009). They also expressed the classic pattern of International Society for Cellular Therapies (ISCT) markers (Table 1). These properties indicate that human endometrium contains a small population of MSC.
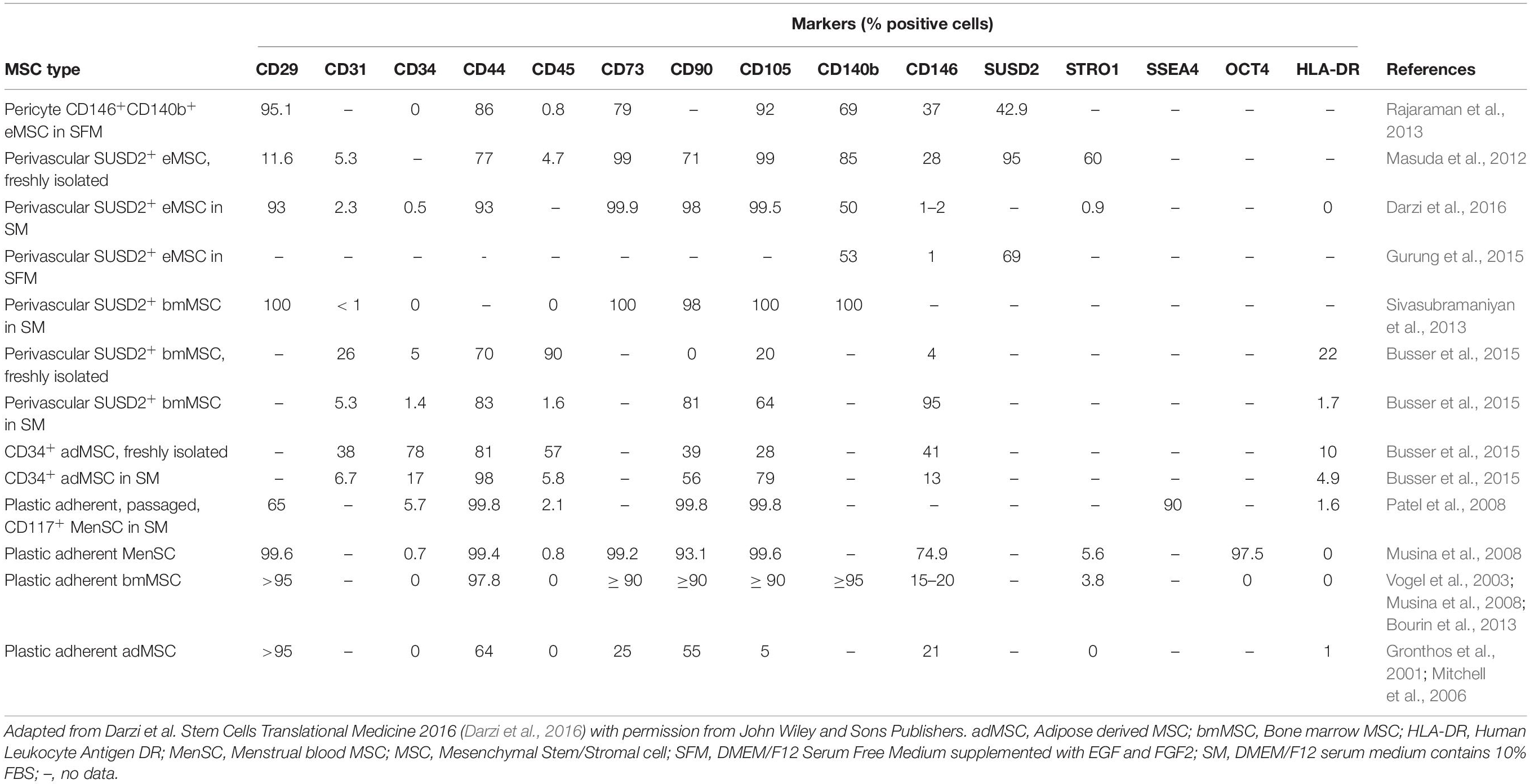
Table 1. Comparison of phenotypic markers of endometrial, menstrual, bone marrow, and adipose tissue MSC isolated by plastic adherence or by SUSD2 or CD34 cell sorting.
Markers of eMSC
eMSC were identified as perivascular cells by comparing cloning efficiencies of endometrial stromal cells purified using flow cytometry for several surface markers used for bone marrow MSC (Figure 2A) (Schwab et al., 2008). Most CFU-F were from cells co-expressing CD140b and CD146 (Schwab and Gargett, 2007). CD140b+CD146+ cells, comprising 1.5% of endometrial stromal cells, were enriched eightfold for CFU-F over unsorted stromal cells and fulfilled the ISCT MSC criteria (Dominici et al., 2006). Their perivascular identity was revealed in both the basalis and functionalis (Figures 2B,J), indicating that CD140b+CD146+ eMSC could be isolated from endometrial biopsies and would be shed in menstrual blood (Darzi et al., 2016). Gene profiling CD14b+CD146+ eMSC versus CD140b+CD146– endometrial fibroblasts showed 762 differentially expressed genes (Spitzer et al., 2012), indicating that perivascular eMSC are distinct from endometrial fibroblasts as for other MSC types (Menard et al., 2020).
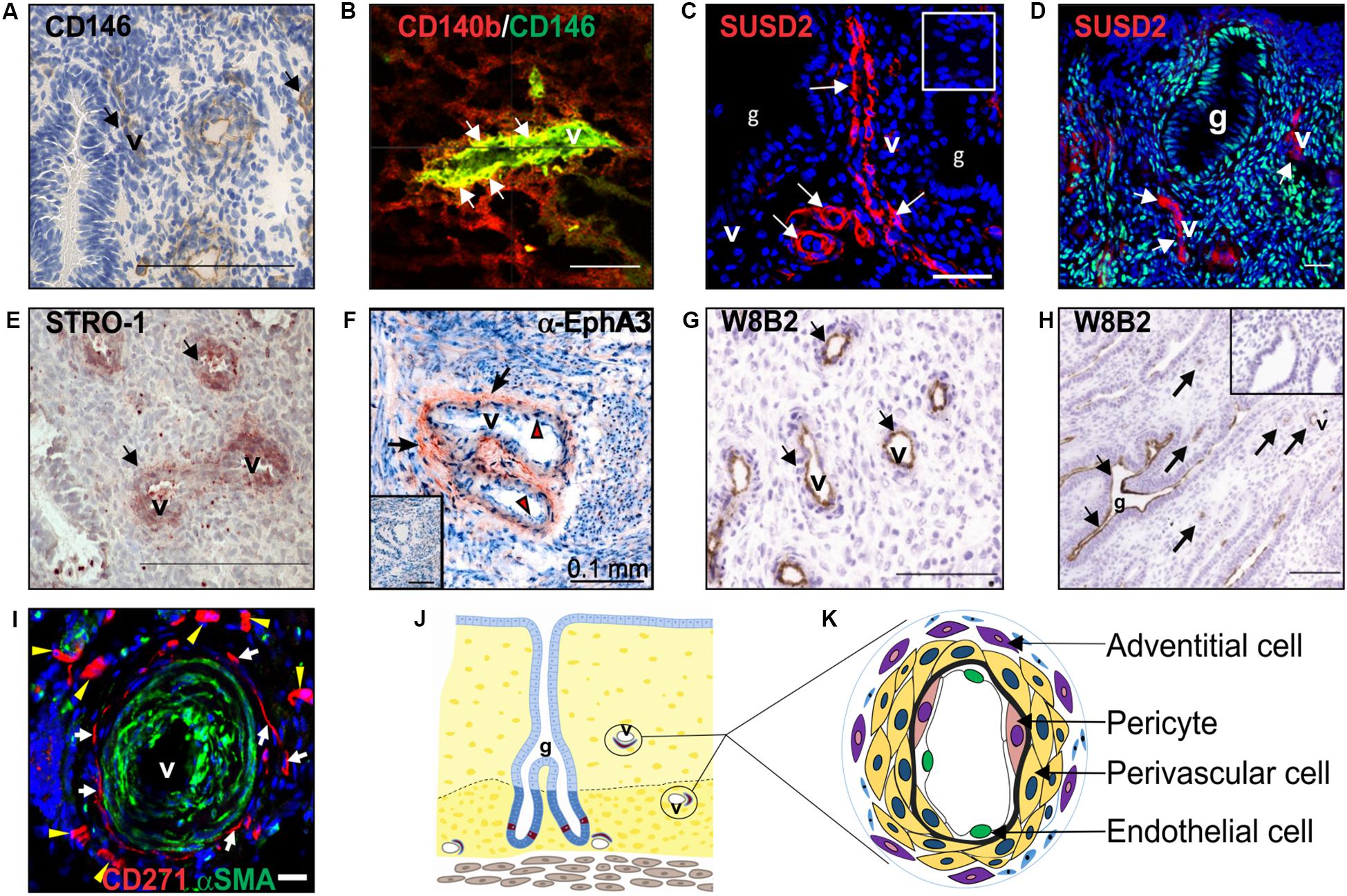
Figure 2. Clonogenic endometrial MSC identified using various surface markers. Surface markers (A) CD146, (B) co-expression of CD140b/CD146, SUSD2 (red) in (C) pre-menopausal and (D) postmenopausal endometrium, showing no colocalization with ERα (green), (E) STRO-1 and (F) EphA3 (red, black arrows), mark clonogenic perivascular eMSC. (G) MSCA-1 (TNAP) detected by the W8B2 antibody is expressed by both perivascular and (H) epithelial cells in the human endometrium. (I) CD271 (red) adventitial perivascular cells mark clonogenic cells in ovine endometrium. (J) Schematic of endometrium showing location of endometrial MSC around the vessels. (K) Schematic enlargement of blood vessels from (J) showing different vascular cell types in the endometrium. Human CD140b+CD146+ cells are pericytes (pink cytoplasm), and SUSD2, STRO-1, and EphA3 cells are perivascular cells (yellow cytoplasm). Ovine CD27+ cells are adventitial cells (violet cytoplasm). Mouse CD34+KLF4+ and LRC cells are also represented by perivascular cells (yellow cytoplasm). Images reprinted with permission from: (A,E) Figures 1E, 4C from Oxford University Press (Schwab et al., 2008), (B) Figure 4I from Oxford University Press (Schwab and Gargett, 2007), (C) Figure 1A from Bioscientifica (Yang et al., 2019), (D) Figure 4A from Oxford University Press (Ulrich et al., 2014b), (F) Figure 1A from Public Library of Science (To et al., 2014), (G,H) Figures 5C,D from Mary Ann Liebert (Sobiesiak et al., 2010), (I) Figure 5D from Public Library of Science (Letouzey et al., 2015) and (K) Figure 2 from Wiley Online Library (Darzi et al., 2016).
Similar to bone marrow MSC, perivascular eMSC are also MSCA-1+ (Sobiesiak et al., 2010), a marker antibody that identifies tissue non-specific aminopeptidase (TNAP), however, this marker is not useful for sorting eMSC as it also marks glandular epithelial cells (Figures 2G,H).
To identify a single marker of perivascular eMSC, endometrial stromal cell suspensions were screened with a panel of perivascular and other novel antibodies by flow cytometry and immunohistochemistry of human endometrium (Masuda et al., 2012). Using this strategy, the W5C5 antibody identified a robust marker of stromal cells from pre- and postmenopausal endometrium with MSC properties, enriching clonogenic cells 18-fold over W5C5– fibroblasts (Masuda et al., 2012; Ulrich et al., 2014b). Sushi Domain-containing 2 (SUSD2) was the antigen identified by the W5C5 antibody (Sivasubramaniyan et al., 2013). SUSD2+ cells comprise 4.1% of endometrial stromal cells and in addition to satisfying the ISCT criteria, they reconstituted stromal tissue in vivo under the kidney capsule of NOD-Scid γ (NSG) mice. Non-ISCT markers also expressed by freshly isolated SUSD2+ eMSC include CD117, CD140b, CD146, and STRO-1 (Figure 2E). More clonogenic cells were present in the SUSD2+CD146+ and SUSD2hi subpopulations than in the CD140b+CD146+ co-expressing population (Masuda et al., 2012). SUSD2 enables prospective isolation of eMSC from freshly isolated cell suspensions using magnetic bead sorting, providing a more clonogenic population than obtained by flow cytometry sorting, which adversely affects cell viability (Masuda et al., 2012). This is an important consideration for clinical translation.
The specific markers of eMSC show that these cells are located around blood vessels in both the functionalis (Figures 1, 2) indicating they are shed into menstrual fluid as the functionalis breaks down during menstruation (Figure 1B). Similarly, stromal fibroblasts are shed into menstrual fluid. Both eMSC and stromal fibroblasts (MenSC) are shed in numbers proportionate to their composition in endometrial functionalis tissue, with eMSC comprising a minority subpopulation. The adult stem cell properties of human eMSC suggest that stromal fibroblasts are their progeny, and to date the only evidence comes from xenografting SUSD2+ eMSC into immunocompromised mice where stromal tissue was generated (Masuda et al., 2012).
Differentiation of eMSC
Physiologically, eMSC around spiral arterioles differentiate into decidual cells under influence of the pregnancy hormone, progesterone, during the secretory stage of the menstrual cycle (Gellersen and Brosens, 2014). This decidual differentiation spreads to the stromal fibroblasts beneath the luminal epithelium. Decidual cells are specialized secretory cells that provide an immunoprivileged environment for an implanting embryo to establish the materno-fetal interface. Subpopulations of eMSC and stromal fibroblasts undergo senescence during the differentiation process (Lucas et al., 2016) and when no embryo implants, progesterone levels fall and menstruation ensues (Figure 1).
Transcriptional profiling of endometrial SUSD2+ eMSC and SUSD2– stromal fibroblasts revealed a distinct gene signature for both cell types following decidual differentiation in vitro (Murakami et al., 2014). Known and novel perivascular genes were upregulated in SUSD2+ eMSC, which produced lower levels of inflammatory mediators and chemokines in vitro compared to SUSD2– stromal fibroblasts. Similarly, the inflammatory gene signature of freshly isolated and cultured CD140b+CD146+ eMSC had fewer transcripts than CD140b+CD146– endometrial stromal fibroblasts (Barragan et al., 2016). Upon decidualization (differentiation) induction SUSD2+ eMSC and SUSD2– stromal fibroblasts showed greater divergence of their respective secretomes, with the eMSC producing much higher levels of leukemia inhibitory factor and the chemokine CCL7 than stromal fibroblasts. These varying features highlight differences between perivascular eMSC and stromal fibroblasts.
Embryologically, endometrium derives from the mesoderm. Thus, it is not unexpected that endometrial MSC and stromal fibroblasts can be induced to differentiate into mesodermal lineages. Differentiation of eMSC into classic mesodermal lineages in vitro as recommended by the ISCT has been shown for clonogenic endometrial stromal cells, SUSD2+ and CD140b+CD146+ cells (Schwab and Gargett, 2007; Gargett et al., 2009; Masuda et al., 2012; Su et al., 2014). Endometrial stromal fibroblasts also show similar mesodermal lineage differentiation, as do bone marrow-derived stromal cells (Haniffa et al., 2009). The reader is referred to table III in Gargett et al. (2016) for a comprehensive list of studies describing mesodermal lineage differentiation of eMSC and endometrial stromal fibroblasts. Differentiation into endodermal lineages include hepatocytes from clonogenic eMSC in vitro (Yang et al., 2014) and insulin-and glucagon-secreting pancreatic lineages both in vitro and in vivo from endometrial stromal fibroblasts (Li et al., 2010; Santamaria et al., 2011). Similarly, cultured endometrial fibroblasts have been differentiated into ectodermal lineages such as dopamine-secreting neurons in vitro and in vivo, where they may have also induced endogenous neural cell secretion (Wolff et al., 2011). Oligodendrocyte progenitor cells have been differentiated from endometrial stromal fibroblasts (Ebrahimi-Barough et al., 2013). It is not known if eMSC differentiate into ectodermal lineages.
Endometrial MSC in Other Species
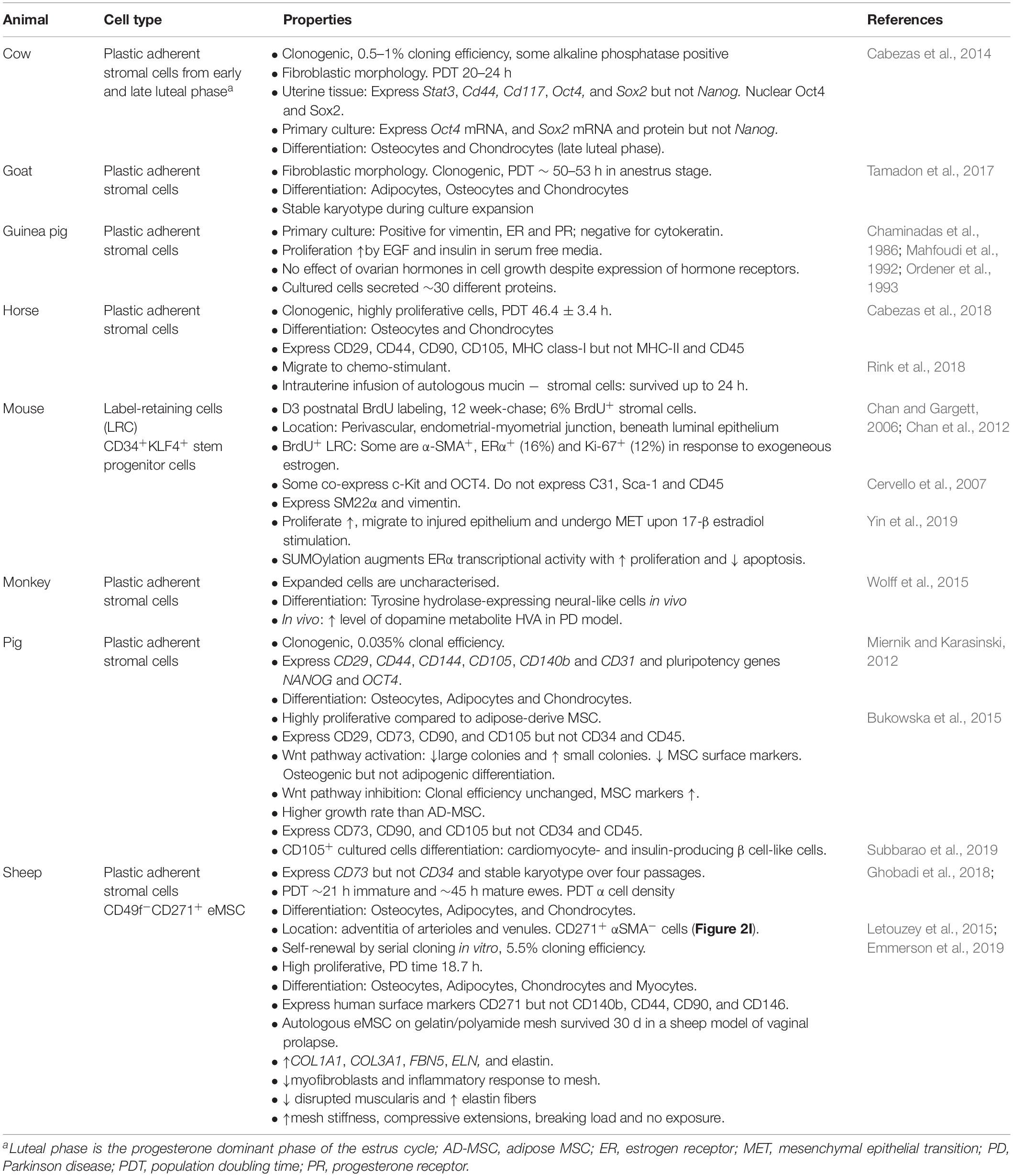
The dynamic nature of the endometrium is not limited to humans. Endometrium of other species also undergo proliferation and differentiation, and endure high levels of regeneration during their reproductive life (Rozemuller et al., 2010; Lara et al., 2018). No animal models can replace humans, however, they provide preclinical models bridging the gap between the in vitro potential of MSC and future clinical use in humans. The identification of endometrial stromal/stem cells in non-human species has made this possible. Mammalian MSC have been identified in the endometrium (Lara et al., 2018) of mice (Chan and Gargett, 2006; Cervello et al., 2007), guinea pig (Ordener et al., 1993), pig (Miernik and Karasinski, 2012; Bodek et al., 2015), sheep (Letouzey et al., 2015; Emmerson et al., 2019), cow (Cabezas et al., 2014), goat (Tamadon et al., 2017), horse (Cabezas et al., 2018) and non-human primates (Padykula et al., 1989; Wolff et al., 2015) (Table 1). Label-retention, plastic adherence or specific surface markers have been used to isolate endometrial MSC and demonstrate properties similar to human eMSC and bmMSC.
Murine Label-Retaining Stromal Fibroblasts
Quiescent stem-like endometrial stromal cells were first identified in mice as bromodeoxyuridine label-retaining cells (LRC), which accounted for 6% of the population after 12 weeks of chase in normal cycling mice (Chan and Gargett, 2006). They were localized adjacent to luminal epithelium, near blood vessels and at the endometrial-myometrial junction, similar to their basalis location in human endometrium (Schwab and Gargett, 2007). Stromal LRCs were both CD31– and CD45–, indicating they were neither endothelial cells or leukocytes, and perivascular LRCs were αSMA+ (Chan and Gargett, 2006). Approximately 16% expressed estrogen receptor-α (ER-α) and were recruited into cell-cycle following estrogen stimulation indicating their involvement in cyclical endometrial regeneration (Chan and Gargett, 2006; Chan et al., 2012). LRCs were also positive for stem cell markers c-Kit and Oct4 (Cervello et al., 2007). However, stem-cell antigen-1 (Sca-1) was not expressed in stromal LRCs (Chan and Gargett, 2006).
Recently, CD34+KLF+ endometrial stromal stem/progenitor cells were identified in the perivascular region in the endometrium of a menstruating mouse model (Yin et al., 2019). They expressed the smooth muscle marker, SM22α, and vimentin and upon estrogen stimulation trans-differentiated into gland-like structures lined with E-cadherin-expressing epithelial cells. SUMO-endopeptidase-1 (SENP1)-deletion in SM22α+cells induced SUMOylation and activation of ERα which promoted SM22α+CD34+KLF4+ cell proliferation and transdifferentiation into endometrial epithelium via cyclin D1. Mice share ∼85% of protein-coding regions with humans and have 99% genetic similarity (NIH, 2010), their small size and the development of transgenic humanized mice allow their utility as cost-effective models. More research on mouse eMSC isolation and characterizationis warranted.
Ovine eMSC
Endometrial stromal fibroblasts have been isolated from Fars native sheep uteri as plastic adherent cells (Ghobadi et al., 2018) or clonogenic cells from Border-Leicester-Marino ewes (Letouzey et al., 2015). Plastic adherent cells expressed CD73 but not CD34, with osteogenic and adipogenic differentiation potential and karyotype stability over four passages. Population doubling times were directly related to the cell seeding density and inversely to age of the ewe (Table 2). Clonogenic stromal cells were enriched using CD271+CD49f– surface markers, showing properties similar to bmMSC and human eMSC, with greater clonogenicity, self-renewal by serial cloning compared to CD271–CD49f– fibroblasts, and differentiated into adipogenic, myogenic, osteogenic and chondrogenic lineages. Apart from CD271, human MSC markers CD44, CD90, CD140b, and CD146 did not cross react with ovine eMSC. The lack of specific ovine marker antibodies has hampered further surface phenotype characterization. Although in a perivascular location, ovine eMSC unlike their human counterpart, were not located in close apposition to vWF+ endothelial cells. Neither did they co-localize with αSMA suggesting they were not pericytes, but rather perivascular adventitial cells, a cell population with similar properties to MSC (Corselli et al., 2012; Crisan et al., 2012). The ability to purify characterized ovine eMSC has enabled the investigation of their use in uro-gynecological disorders such as pelvic organ prolapse (POP) (Letouzey et al., 2015; Emmerson et al., 2019). The domestic ewe has proven a good large animal model because they develop spontaneous POP after vaginal delivery with incidence increasing with parity, similar to women (Couri et al., 2012; Young et al., 2017). Autologous ovine eMSC in a gelatin hydrogel applied onto a polyamide scaffold and implanted into the vagina of the ovine POP model survived for at least 30 days, modulated the inflammatory response, promoting good tissue integration with no postoperative mesh-exposure (Emmerson et al., 2019), one of the main complications associated with human transvaginal mesh (Frankman et al., 2013).
Non-human Primate Endometrial Stromal Cells
Endometrial-derived stromal cells from the non-human primate, green monkey engrafted and differentiated into neuron-like cells when injected into the striatum of males with 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) induced Parkinson’s disease (Wolff et al., 2015). The expanded cells used expressed CD140b, CD146, and CD90, but were otherwise not characterized (Wolff et al., 2015), possibly due to lack of suitable antibodies. The differentiated cells expressed tyrosine hydrolase, a rate limiting enzyme for L-DOPA synthesis, proliferated and produced dopamine metabolites indicating their potential use in cell-based therapies (Wolff et al., 2015). Although non-human primates are ideal for simulating human diseases due to their phylogenetic similarity, their utility in research raises ethical, practical and financial issues.
Menstrual Blood MSC (MenSC)
Menstrual blood is a readily accessible, non-invasive source of large numbers of endometrial stem/stromal cells (MenSC) (Ulrich et al., 2013; Darzi et al., 2016), easily collected using a menstrual cup (Figure 1B) (Musina et al., 2008; Patel et al., 2008; de Carvalho Rodrigues et al., 2012). Menstrual blood contains fragments of shedding endometrial tissue (Figure 1C) which is cultured directly onto plastic similar to bmMSC. Adherent MenSC have typical stromal fibroblast morphology and rapidly propagate with a doubling time of 18–36 h (Meng et al., 2007; Patel et al., 2008). MenSC have higher proliferative capacity, 30–47 PD before senescence, compared to bmMSC, which are generally limited to ∼20 PD (Cui et al., 2007; Allickson et al., 2011). MenSC yield is 2–4-fold higher compared to bmMSC (Alcayaga-Miranda et al., 2015a). As for other adult stem cells, the lifespan of MenSC is relatively short in comparison with human embryonic stem cells (hESC). MenSC only maintain 50% of their telomerase activity at passage 12 compared to hESCs. However, MenSC have more telomerase activity than bmMSC (Patel et al., 2008), perhaps due to the regenerative capacity of the endometrial stroma.
Besides shed endometrial tissue fragments, menstrual fluid also contains peripheral blood. The source of MSC in menstrual fluid could be from bone marrow as well as endometrium. However, peripheral blood contains exceedingly rare bmMSC, as demonstrated by CFU-F analysis. Just 2 CFU-F were found in peripheral blood from 10 patient samples at a frequency of 0–1 CFU-F per 4 × 107 nucleated cells (Kuznetsov et al., 2001). In contrast, menstrual fluid from 18 healthy women contains 600 CFU-F per ml (Alcayaga-Miranda et al., 2015a), suggesting that these CFU-F are derived from the endometrial fragments rather than circulating bone-marrow-derived MSC. Rigorous lineage tracing in chimeric mouse models has also shown that bone marrow stem cells do not contribute to endometrial stromal lineages (Ong et al., 2018). Thus MenSC, comprising mainly endometrial stromal fibroblasts and a small proportion of eMSC found in menstrual fluid are derived from sloughing endometrial tissue rather than non-shedding bone marrow stroma.
Markers of MenSC
MenSC possess the classic ISCT bmMSC markers and are positive for HLA-ABC, negative for HLA-DR and do not express hematopoietic lineage markers (Cui et al., 2007; Meng et al., 2007; Darzi et al., 2012; Kazemnejad et al., 2012; Khanjani et al., 2014; Khanmohammadi et al., 2014). MenSCs, similar to clonogenic eMSC, differ from bmMSC as they do not express STRO-1 (Cui et al., 2007; Meng et al., 2007; Patel et al., 2008; Schwab et al., 2008; Khanmohammadi et al., 2014). Another difference is that MenSCs highly express cytoplasmic OCT-4 (Borlongan et al., 2010) which is not expressed by eMSC (Gurung et al., 2015; Gurung et al., 2018b) or conventional bmMSC. Several inconsistencies in MenSC phenotype have been observed for pluripotency markers c-KIT and SSEA-4. Some have reported these markers in isolated MenSCs (Patel et al., 2008; Borlongan et al., 2010), while others were negative (Cui et al., 2007; Hida et al., 2008; Musina et al., 2008; Khanjani et al., 2015). Others observed a different pattern of these markers in isolated MenSC (Meng et al., 2007; Musina et al., 2008; Darzi et al., 2012; Kazemnejad et al., 2012) including expression of SSEA-4 and/or NANOG in some c-KIT+ cells from cultured menstrual blood (Meng et al., 2007; Patel et al., 2008; Borlongan et al., 2010). Heterogeneity of MenSs cultures may explain these disparities, resulting from differences in menstrual blood sampling day, collection technique and enrichment protocol.
Mesodermal Lineage Differentiation
MenSC have been induced to differentiate into mesodermal lineages characteristic of bmMSC (Table 3) to satisfy the minimal criteria for MSC. Similar to marker expression, the degree of MenSC differentiation into mesodermal lineages varies and is dependent on the isolation method (Meng et al., 2007; Darzi et al., 2012; Kazemnejad et al., 2012; Khanjani et al., 2014; Khanjani et al., 2015). MenSCs isolated by density gradient centrifugation and plastic adherence have lower capacity to differentiate toward osteoblasts compared to bmMSC using both cytochemical and molecular analyses (Darzi et al., 2012). In contrast, c-KIT+ MenSC possessed similar differentiation capacity to bmMSC (Patel et al., 2008), although, Alizarin red alone without quantification was used. It is unknown whether c-KIT-sorted MenSC have osteogenic differentiation ability.
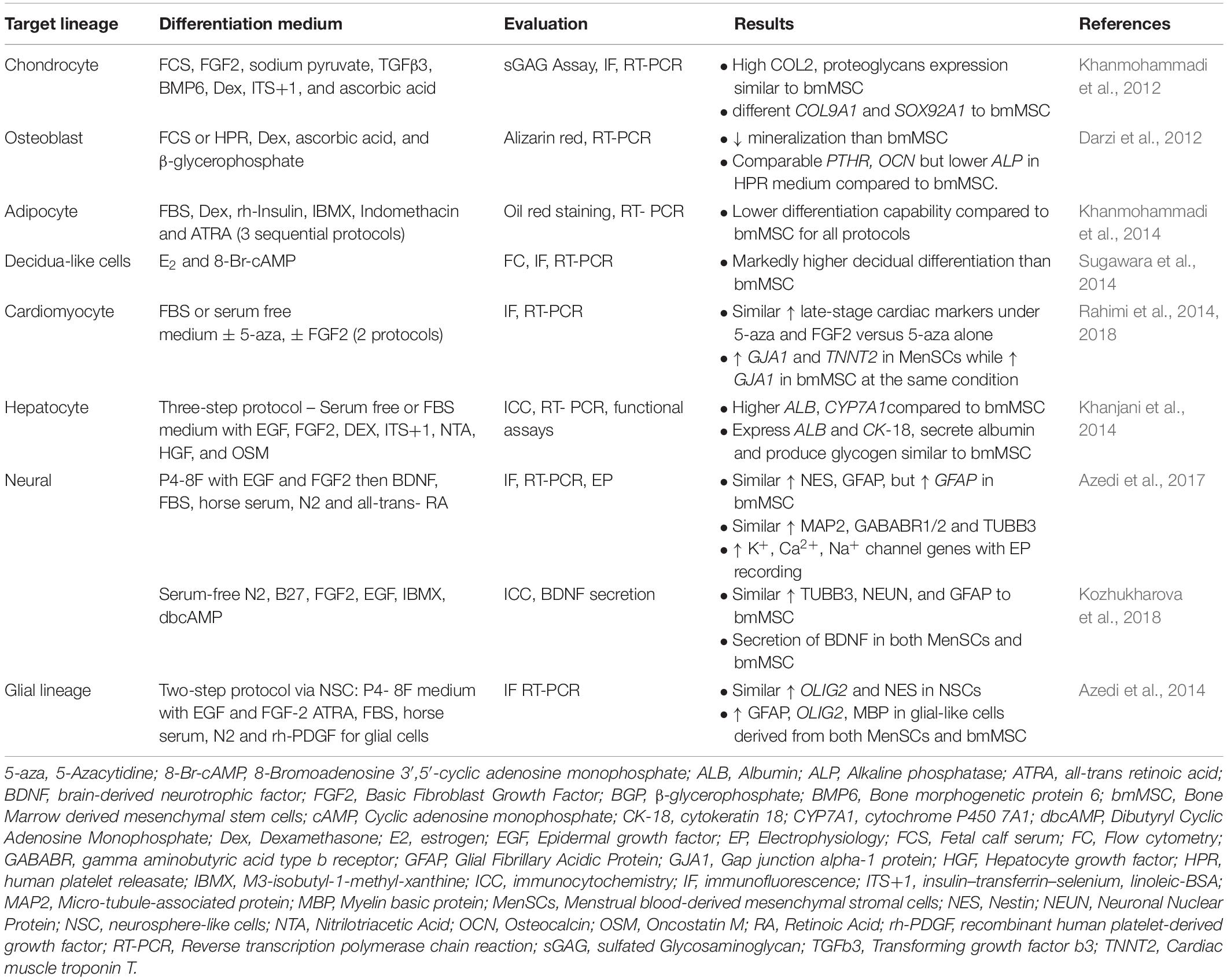
Table 3. Comparison of in vitro differentiation capability of menstrual blood- versus bone marrow-derived mesenchymal stem/stromal cells.
Adipogenic differentiation of MenSC isolated by density gradient centrifugation and plastic adherence is limited when assessed by Oil-red O staining (Musina et al., 2008) and significantly lower than umbilical cord MSC (Jin et al., 2013) and bmMSC (Khanmohammadi et al., 2014). Fortification of adipogenic induction medium with rosiglitazone promoted MenSC differentiation into adipocytes as analyzed by molecular and cytochemical techniques (Khanmohammadi et al., 2014). Purifying cultured MenSC using the c-KIT (CD117) marker resulted in high adipogenic differentiation capacity (60–70%) (Patel et al., 2008), likely due to a more homogeneous MSC population, although it is unknown if CFU-F are also purified in the c-KIT+ subpopulation of cultured MenSC.
Differentiation of MenSC into the chondrocytic lineage has been demonstrated for CD117 purified cells in 2D cultures yielding 45% positive cells, similar to bmMSC (Patel et al., 2008). Unfractionated MenSCs cultured in a 3D nanofibrous scaffold enhanced chondrogenic commitment compared to a 2D culture system (Kazemnejad et al., 2012), with extensive cartilage-like extracellular matrix containing ∼50% more glycosaminoglycan than control MenSCs differentiated in 2D. Chondrogenic differentiation requires very low O2 tension, which is better established in 3D compared to 2D cultures. MenSCs produce high levels of Activin A, IGF-1, and FGF2, key growth factors involved in chondrogenesis (Ren et al., 2016; Uzieliene et al., 2018).
Co-culture of MenSC with murine cardiomyocytes generated spontaneously beating human cells expressing cardiac specific markers, indicating their differentiation into troponin T-expressing cardiomyocytes (Hida et al., 2008). In vitro cardiac differentiation of MenSC was compared with bmMSC using two differentiation protocols and showed that continuous FGF2 was superior to 5-aza-2′-deoxycytidine alone, as shown by increased levels of late-stage cardiac markers (connexin 43 and Troponin T) (Table 3). This suggests that FGF2 has a key role in differentiating cardiac cells from MSC sources. MenSC had greater capacity than bmMSC to differentiate toward cardiomyocytes regardless of the differentiation protocol (Rahimi et al., 2014).
Collectively, the differentiation capability of unfractionated MenSC isolated using conventional methods into two mesodermal lineages (osteoblasts and adipocytes) is lower than bmMSC isolated in a similar manner. This suggests differences in tissue specific MSC properties likely reflecting their tissue of origin. Purification of the rarer perivascular MSC with specific markers appears to improve MenSC differentiation, although c-KIT is not expressed on perivascular cells of human endometrium in vivo (Figure 2), but whether induced in cultured perivascular eMSC is unknown.
Endometrial stromal fibroblasts naturally differentiate into decidualized stromal cells in vivo. Decidualization is mediated by estrogen and progesterone, and supports the establishment and continuation of pregnancy. MenSC have been differentiated into decidual-like cells with 8-Br-cAMP and progesterone, showing morphological changes and increased expression of the decidualization markers prolactin and insulin-like growth factor binding protein-1 (IGFBP-1), and attenuated expression of MSC surface markers (Sugawara et al., 2014). Greater secretion of these decidual proteins was observed for MenSC compared to bmMSC and amnion MSC (Domnina et al., 2016). It is possible that easily obtained MenSC could be differentiated into decidual-like cells as a potential strategy to mitigate infertility associated with insufficient endometrial decidualization.
MenSC Differentiation Into Ectodermal and Endodermal Lineages
Cultured MenSC transdifferentiate across lineage boundaries into ectodermal (neurons and glia), and endodermal (hepatocyte) lineages (Table 3). c-KIT sorted MenSCs express neuronal phenotypic markers when grown in appropriately conditioned medium (Borlongan et al., 2010). MenSC formed neurosphere-like cells and then differentiated into neural and glial-like cells comparable to bmMSC, and shown by increased expression of classic neural markers (nestin, microtubule-associated protein 2, gamma-aminobutyric acid type B receptor subunit 1 and 2, and tubulin β3 class III) (Table 3) (Azedi et al., 2014, 2017).
Differentiation of MenSC into hepatocytes is dependent on the concentrations of hepatocyte growth factor (HGF), oncostatin M (OSM) and removal of serum from the induction medium (Khanjani et al., 2015). Up-regulation of albumin and CYP7A1 expression was higher in MenSC- compared to bmMSC-derived hepatocyte-like cells, although cytokeratin-18 expression, albumin production and glycogen accumulation were either lower or not different (Khanjani et al., 2014), indicating similarity between MenSCs and bmMSC hepatocyte-like cell differentiation potential.
Unfractionated MenSC have potential to differentiate into keratinocyte-like cells, generating epidermal lineage markers via co-culturing with keratinocytes derived from the foreskin of healthy newborns (Faramarzi et al., 2016; Akhavan-Tavakoli et al., 2017). MenSC were also induced to differentiate into keratinocyte-like cells in 3D culture with human foreskin-derived keratinocytes on a bilayer scaffold composed of amniotic membrane and silk fibroin (Arasteh et al., 2018; Fard et al., 2018). The MenSC-derived keratinocytes expressed keratinocyte-specific markers K14, p63 and IVL (involucrin). Generating keratinocytes from MenSC on an efficient natural construct has potential applicability for MSC-based skin wound healing and regeneration (Arasteh et al., 2018; Fard et al., 2018), although these tissue engineering constructs have yet to be evaluated in an animal skin wound repair model.
MenSC show considerable capacity to differentiate into numerous lineages in vitro, although have lesser ability for several bmMSC lineages. It would now be important to demonstrate the potential of MenSC to undergo differentiation into these lineages in vivo in animal models. Genomic sequencing (e.g., RNAseq) would also shed light on how far down the various lineages MenSC differentiate.
Immunomodulatory Properties of eMSC and MenSC
Since the first report on the immunomodulatory properties of MSC in 2002 (Bartholomew et al., 2002), numerous studies have shown significant impact of MSC on key cells of the innate and adaptive immune systems (Le Blanc and Mougiakakos, 2012; Wang et al., 2014). These non-stem cell properties have been exemplified in bmMSC (da Silva Meirelles et al., 2006). MSC regulate monocyte infiltration to the site of injury, macrophage polarization and dendritic cell maturation (Jiang et al., 2005; Kim and Hematti, 2009). MSC also inhibit NK cell proliferation and target cell killing, and secrete IFN-γ (Sotiropoulou et al., 2006). In the adaptive immune system, MSC suppress T cell proliferation, shifting Th17 cells toward T regulatory cells (Tregs) (Di Nicola et al., 2002; Sato et al., 2007). MSC also impede B cell proliferation (Augello et al., 2005; Corcione et al., 2006). Mechanism of interaction relies on direct cell-cell contact and/or MSC secretion of immunosuppressive factors including Indoleamine 2,3 deoxygenase (IDO), prostaglandin E2 (PGE2), nitric oxide (NO), human leukocyte antigen G5 (HLA-G5), IL-10, IL-6 and TGF-β (Di Nicola et al., 2002; Ren et al., 2010). MSC have also been used to successfully treat patients with severe immune disorders, including Graft-Versus-Host Disease (GVHD) and Crohn’s disease (Dalal et al., 2012). This extensive literature indicates that MSC have immunosuppressive rather than immunostimulatory functions. Here we review the literature on the immunomodulatory properties of eMSC and MenSCs.
In vitro Immunomodulatory Properties of eMSC
The immunomodulatory properties of perivascular eMSC have only recently been investigated. A transcriptional study on CD140b+CD146+ eMSC revealed that perivascular eMSC differentially expressed several immunomodulatory genes compared to endometrial stromal fibroblasts (Spitzer et al., 2012) (Table 4). A similar gene profile of differentially expressed genes was observed between SUSD2+ perivascular eMSC and SUSD2– stromal fibroblasts (Murakami et al., 2014) (Table 4), including anti-inflammatory IL10. The gene profile of perivascular eMSC treated and non-treated with the TGFβ receptor (TGFβR) inhibitor, A83-01 to prevent apoptosis and senescence during culture expansion, revealed upregulation of many immune response genes in treated cells, including interleukins (IL15, IL33, IL6ST), TNF and IFNγ related genes, PGE2 synthesis genes (PLA2G4A, PTGS2/COX-2, PTGES), and Toll-like receptors, TLR2 and TLR3 (Gurung et al., 2018b). Together, these finding suggest that eMSC have the ability to interact with the immune system in a similar manner to bmMSC, exerting immunosuppressive functions through paracrine mechanisms and by direct contact with the target cell.
eMSC also influence T cell function by suppressing ConA-stimulated murine T lymphocyte proliferation in a dose-dependent manner (Yang et al., 2019). Blocking the TGFβR in eMSC reversed the immunosuppressive effects of eMSC on T cell proliferation, indicating a role for the TGFβ signaling pathway. Of the various mechanisms assessed, neither IL-10, PGE2, TGFβ or Tregs mediated the eMSC anti-proliferative effects on T cells, however, IL-17A and Dickkopf-1 (DKK-1) were secreted and may be candidates involved. Systemic eMSC failed to inhibit swelling in a T cell-mediated mouse model of skin inflammation, suggesting that eMSC have distinct immunomodulatory properties that may not be sufficient to restrain some T cell-mediated events (Yang et al., 2019).
eMSC Effects on Foreign Body Response to Implanted Scaffolds in vivo
The immunomodulatory role of perivascular eMSC has been explored in animal models of foreign body response to the implanted biomaterials (Table 4). In a nude rat wound healing model, human eMSC seeded on a gelatin-coated polyamide mesh initially increased inflammatory M1 macrophages at the mesh-tissue interface, followed by reduced inflammation around the mesh filaments by switching macrophages from an M1 to a wound healing M2 phenotype. Long term, there were reduced CD68+macrophages at the mesh tissue interface (Ulrich et al., 2014a). Human eMSC seeded on the same polyamide/gelatin mesh inhibited secretion of the inflammatory cytokines IL1β and TNF in early stages of the host response, whereas induced gene expression of anti-inflammatory M2 markers was observed later (Darzi et al., 2018). These responses occurred earlier and were more marked in the immunocompetent than immunocompromised mice. This favorable immunosuppression by eMSC was confirmed subsequently by eMSC-seeded degradable poly(l -lactic acid)-co-poly(ε-caprolactone) (PLACL) nanofibers and 3D bio-printed tissue engineering constructs in the same wound healing immunocompetent mouse models (Mukherjee et al., 2019b; Paul et al., 2019).
In vitro Immunomodulatory Properties of MenSC
The immunomodulatory properties of MenSC have been also been investigated, often in comparison with bmMSC. MenSC effects have been mainly studied on T cell responses. In mixed lymphocyte reactions (MLR), comprising MenSCs mixed with allogenic human peripheral blood mononuclear cells (PBMCs), cellular proliferation, IFN-γ and TNF levels were suppressed, while IL-4 production increased (Murphy et al., 2008). The MenSC suppressive effect on the allogenic MLR was dose-dependent and biphasic, with a high MenSC:PBMC ratio (1/2) suppressing PBMC proliferation and a low ratio (1/32) supporting proliferation (Nikoo et al., 2012). MenSC effects on cytokine levels were similarly concentration dependent with reduced anti-inflammatory IL4+IL10+CD4+ T cells at low MenSC:PBMC ratio compared to bmMSC (Luz-Crawford et al., 2016). These effects suggest distinct immunosuppressive activity of MenSC, similar to eMSC and appear less than bmMSC. This may be due to more HLA-DR molecules on the MenSC surface and fewer IFN-γ receptors, and that MenSC produce less IDO, COX2, and Activin A (Luz-Crawford et al., 2016) compared with bmMSC.
Pretreatment (licensing) of bmMSC with an inflammatory stimulus enhances their immunosuppressive properties. In contrast to the anti-proliferative effect of IFN-γ-pre-treated bmMSC on CD4+ T cells, IFN-γ-IFN-g-pre-treated MenSC show a milder response (Aleahmad et al., 2018). Untreated MenSC cocultured with CD4+ T cells at ratios of 1:2–1:8 had a pro-inflammatory effect, increasing the proliferation of anti-CD3/CD28-acitivated T cells, and IFN-γ pretreatment only partially reduced this effect, suggesting that IFN-γ responsiveness of MenSC is comparatively lower than bmMSC (Figure 3) (Aleahmad et al., 2018). The impact of MenSC on human T cell proliferation in vitro is complex and depends on the cytokine milieu, T cell stimulating factors, MenSC/T cell ratio and the co-culture system. MenSC have a weaker and distinct in vitro effect on T cell proliferation compared to bmMSC (Aleahmad et al., 2018), possibly similar to the lesser characterized eMSC responses.
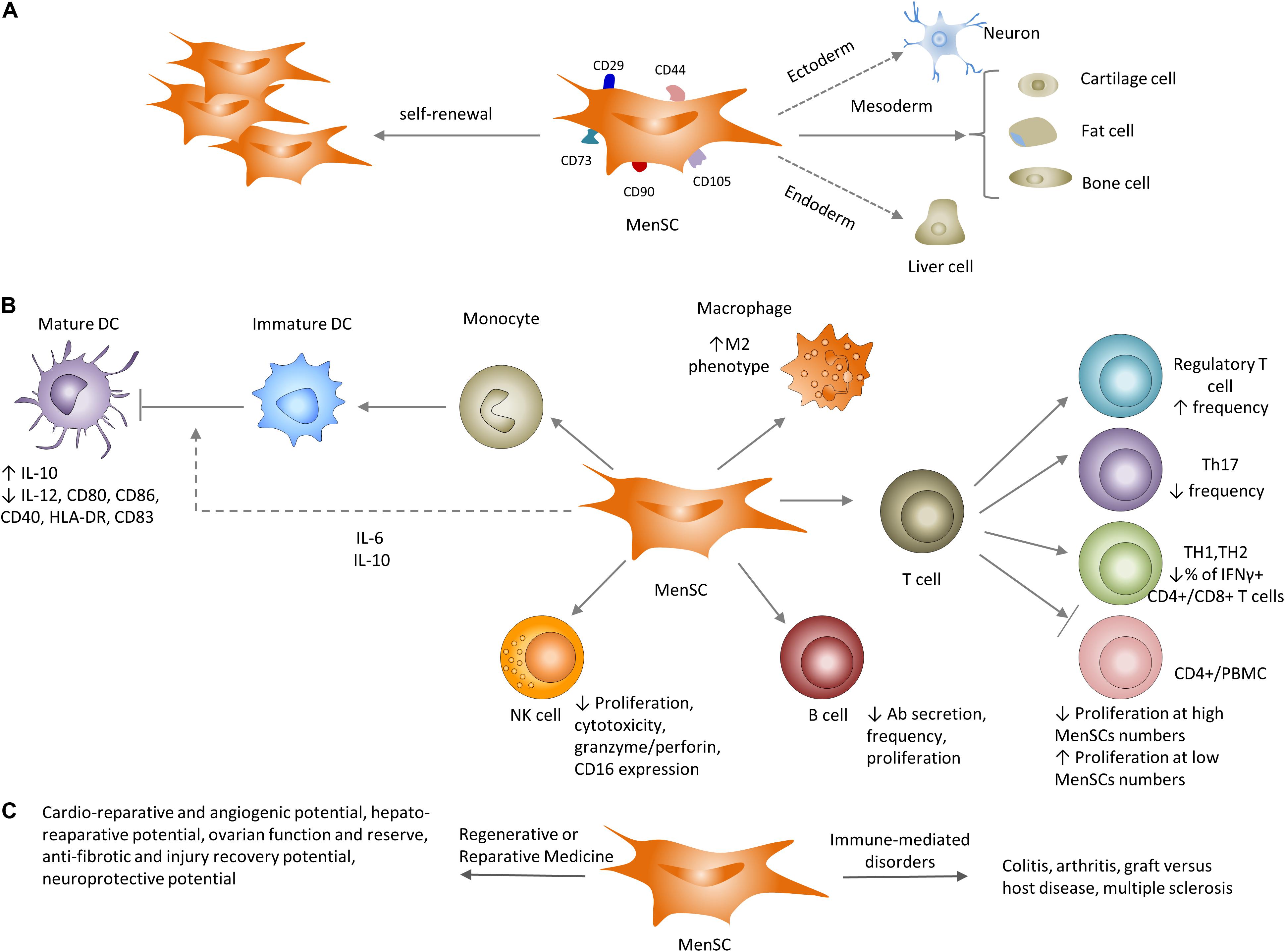
Figure 3. Properties of MenSCs in vitro and in vivo. (A) Similar to their well-known counterparts (bmMSC), MenSCs have the ability to differentiate to cells of different lineages and express typical MSC-associated markers. (B) Impact of MenSCs on the adaptive and innate immune systems. MenSCs secrete IL-6 and IL-10 that inhibit optimal maturation of human monocyte-derived dendritic cells (DC). MenSCs shift macrophages toward the M2 (anti-inflammatory) profile. MenSCs either inhibit or support T cell proliferation depending on MenSC/T cell (PBMC) ratio. MenSCs increase Treg frequency, decrease the number of TH17 and IFNγ+ CD4+/CD8+ T cells and cytotoxic capacity of NK cells. MenSCs decrease antigen-specific antibody secretion and proliferation of B cells. (C) Utilization of MenSCs in preclinical settings ranges from immune-mediate disorders: arthritis and GVHD to regenerative and reparative applications: cardio protection, liver and lung fibrosis and neuroprotection.
As expected, MenSC also have an impact on the innate immune system, including human blood monocyte-derived dendritic cells (MoDCs) (Table 4) (Bozorgmehr et al., 2014), NK cells and tissue macrophages. MenSC co-cultured with human monocytes interfered with MoDC differentiation at the phenotypic level. The immature DCs expressed suboptimal co-stimulatory molecules and had low levels of CD40, CD80, CD83 and CD86. MenSC secreted IL-6 and IL-10 (Bozorgmehr et al., 2014), which typically inhibit monocyte to DC differentiation (Allavena et al., 1998; Chomarat et al., 2000). Uterine NK (uNK) cells constitute the main component of the endometrial innate immune system, comprising 50–70% of lymphoid cells in early pregnant endometrium (Moffett-King, 2002). uNK cells function in maintaining a successful pregnancy by preventing allorejection of the embryo and regulating vascular remodeling (Hanna et al., 2006; Kalkunte et al., 2009). Dysregulation of uNK cell function may be involved in the pathogenesis of recurrent pregnancy loss (Dosiou and Giudice, 2005) indicating the importance of tightly regulating uNK cell cytotoxic function. Unstimulated MenSCs induced NK cell proliferation partly due to lower production of IGFBPs 1-4 compared to bmMSC. However, in a pro-inflammatory milieu involving IFN-γ/IL-1β, MenSCs substantially inhibited NK cell proliferation through production of IL-6 and TGF-β (Shokri et al., 2019). IFN-γ/IL-1β-stimulated MenSC also curbed NK cell cytotoxicity by decreasing granzyme A, granzyme B, and perforin expression. NK cells also killed MenSC in a MHC- and time-dependent manner. These results suggest a critical role for MenSC in endometrial tissue homeostasis and induction of a pregnancy-friendly phenotype in decidual NK cells.
MenSC Immunomodulatory Effects in vivo
Recently it was shown that MenSC influence the humoral immune responses in a mouse model of heart transplantation by attenuating antibody responses (Xu et al., 2017). MenSC injection 24 h following allograft heart transplantation prolonged graft survival in recipient mice by rapidly reducing intragraft deposition of donor-specific IgG and IgM antibodies and reducing donor-specific antibody secreting B cells. It remains to be investigated whether MenSC ameliorate autoantibody production in patients with antibody-mediated autoimmune diseases.
In a murine model of colitis, intravenous MenSC injected 2–8 days after disease induction (Cabezas et al., 2014) increased intra-colon IL-4 and IL-10 and decreased IL-2 and TNF, and splenic dendritic cells expressed lower levels of MHC-II compared to untreated controls (Cabezas et al., 2014). The percentage of CD3+CD25+ and CD3+CD8+ T cells was reduced and Tregs increased. MenSC also promoted F4/80+CD206+ M2 macrophage migration and reduced IgG deposition in the injured colon, decreased splenic plasma cells, increased regulatory B cells (Xu et al., 2018), and appeared to mediate these immunomodulatory effects via PD-L1 (Shi et al., 2019).
Not all studies have corroborated the immunomodulatory effects ofMenSC in disease models. MenSC failed to switch T cell-related immune responses toward an anti-inflammatory direction in a murine model of arthritis (Luz-Crawford et al., 2016) (Table 5). MenSC injection increased splenic TNF levels and did not reduce lymph node pro-inflammatory Th17 cells and in contrast, bmMSC did not exert any beneficial impacts on disease progression. MenSC injection into a humanized GVHD mouse model, where irradiated NOD-SCID mice received human PBMC, has been efficacious in reducing disease severity (Luz-Crawford et al., 2016). In this acute inflammatory model, intra-peritoneal MenSC were superior to bmMSC, in contrast to the arthritis model. MenSC improved intestine structure and survival rate. However, immunosuppressive functions were not observed for MenSC, but rather their effect was attributed to their regenerative capacity. MenSCs expressed high levels of VEGF and FGF2, and induced greater vascularization compared with bmMSC. MenSC also had a greater migratory capacity to target organs due to their higher expression of surface CXCR4.
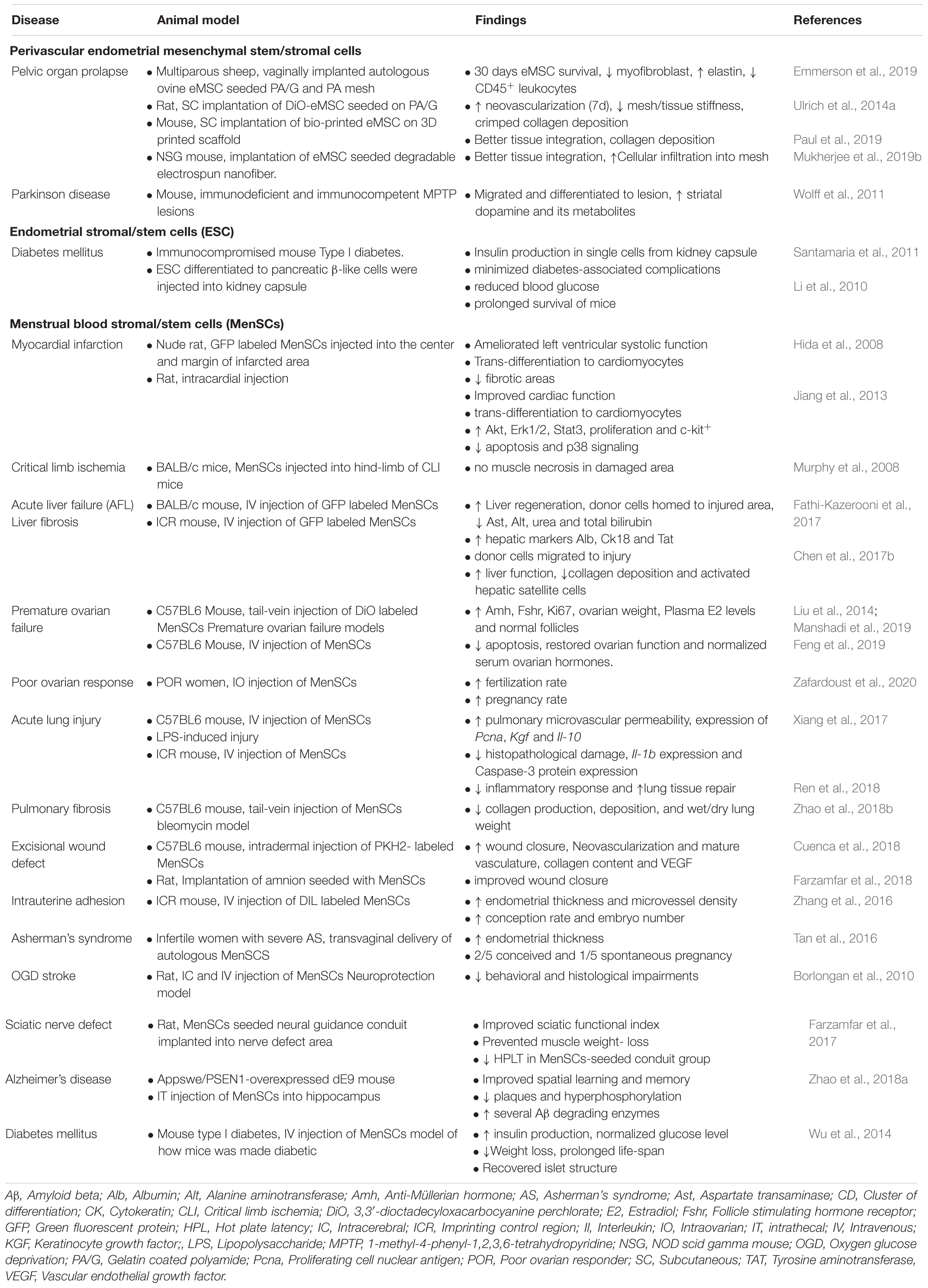
Table 5. Preclinical and clinical applications of endometrial MSC and Menstrual blood stromal cells.
MenSC also have anti-microbial activity. In a cecal ligation and puncture mouse model of sepsis, the combination of MenSC and antibiotic improved the survival rate of affected animals (up to 95%) compared with control animals receiving either treatment alone. The MenSC/antibiotic combination increased bacterial clearance from blood, and reduced the inflammatory cytokines IL-8, TNF, and MCP in peritoneal fluid, without loss of T and B lymphocytes (Alcayaga-Miranda et al., 2015b).
Taken together, eMSC and MenSC have a range of effects on both arms of the innate and specific immune responses (Figure 3), however, the discrepancies between in vitro and in vivo findings and considerable variation between experimental models necessitate further investigation to identify the underlying mechanisms that orchestrate the cross-talk that MenSC and eMSC utilize to modulate the immune system. Standardization of key variables of human disease and disease models including immune versus non-immune nature of the disease, local versus systemic administration of cells, chronic versus acute disease, and the dose and timing of injected MenSC as well as MSC cell type (perivascular versus unfractionated stromal fibroblast populations) and experimental models as has been promulgated for bmMSC will further increase understanding of the distinct properties of eMSC and MenSC.
Therapeutic Potential of eMSC and MenSC
Challenges for MSC-Based Therapies in Regenerative Medicine
Hundreds of clinical trials of various MSC have overall shown underwhelming results in regenerative medicine applications (Prockop et al., 2014). This is due in part to the use of preclinical mouse studies using syngeneic mice, MHC-matched cells and achievable dosing (Galipeau and Sensebe, 2018). Large animal preclinical models are particularly important to trial MSC before going to the clinic to resolve these issues. To date long term engraftment of substantial numbers of infused MSC has not been demonstrated, rather only a small population (2–10%) remain in the days following administration (Dimmeler et al., 2014). Allogeneic MSC will be rapidly removed by the innate immune system, and while autologous cells may prevail, few appear to integrate into tissues. This lack of MSC integration is due to the ischemic or inflammatory environment of the diseased tissue/organ and loss of vascular niches which may be replaced with fibrosis. Aged tissues and chronic disease can also hinder MSC integration (Dimmeler et al., 2014). Despite these challenges, MSC can have dramatic effects through their paracrine actions, appearing to reset the innate immune system and promoting endogenous cellular repair without substantial integration (Caplan, 2016).
eMSC in Regenerative or Reparative Medicine
Since perivascular eMSC originate from a cyclically regenerating tissue with accompanying angiogenesis (Gargett and Rogers, 2001) they are excellent candidates with therapeutic potential for tissue repair and possibly tissue regeneration. Their pericyte and perivascular identity indicate their specific roles in regulating angiogenesis, inflammation and fibrosis (Thomas et al., 2017), suggesting eMSC are a good candidate for regenerative or reparative medicine.
Endometrium and Decidua Regeneration
As mentioned above, human eMSC differentiate into decidual cells. This property has been harnessed for generating a tissue engineered uterus through repopulating decellularized extracellular matrix (ECM) constructs with cells, including endometrial stromal fibroblasts (Fu et al., 2014; Hellstrom et al., 2017; Tiemann et al., 2020). This bioengineering approach has been conducted in decellularized uterus from rat (Miyazaki and Maruyama, 2014; Hellstrom et al., 2016; Kuramoto et al., 2018), pig (Campo et al., 2017) and human (Olalekan et al., 2017). Allogeneic endometrial stromal cells and bmMSC seeded into decellularized rat uteri diffused into the matrix and expressed the stromal marker vimentin and endothelial marker CD31, demarcated by cytokeratin expressing epithelial cells (Miyazaki and Maruyama, 2014). Transplantation of these uterine constructs into partially excised uteri, regenerated endometrial tissue which differentiated into desmin-expressing decidual-cells and achieved pregnancies (Miyazaki and Maruyama, 2014). There was evidence of fetal development, however, no evidence of placentation at the graft site. In another approach to reconstitute native, functional endometrium, a rat GFP+ endometrial epithelial cell-sheet was layered on two layers of adherent GFP+ endometrial stroma cell-sheets and transplanted into a full thickness endometrial defect in a rat model (Kuramoto et al., 2018). Full thickness endometrium with luminal and glandular epithelium and stromal compartments was generated and pregnancy established on the regenerated endometrium with placentation and fetal heart movements detected. Preliminary results using similar approaches in a larger pig model have commenced (Campo et al., 2017). Using human endometrial Side Population cells including stromal cells demonstrated integration of vimentin+ and cytokeratin+ cells in the decellularized pig uterus. In an in vitro model, endometrial stromal cells, which likely contained a small population of eMSC, repopulated decellularized human endometrial substrates and proliferated in the scaffold (Olalekan et al., 2017). Most importantly, the cells underwent decidual changes upon stimulation with an estrogen and progesterone treatment protocol mimicking the human 28-day menstrual cycle. The infiltrated cells expressed estrogen and progesterone receptors, and secreted prolactin and IGFBP-1, markers of decidualization. These recent approaches indicate the feasibility of using eMSC and endometrial stromal fibroblasts as cell sources for regenerative medicine in uterine biology.
Pelvic Organ Prolapse
The potential of eMSC in regenerative or reparative medicine has been explored for a common women’s gynecological disorder, pelvic organ prolapse (Ulrich et al., 2013; Emmerson and Gargett, 2016; Gargett et al., 2019; Mukherjee et al., 2019a). An autologous approach has been investigated and tissue engineering constructs comprising eMSC and novel non-degradable mesh (Ulrich et al., 2012), degradable nanofiber mesh (Mukherjee et al., 2019b), and 3D bioprinted mesh/eMSC (Paul et al., 2019) have been explored in both rodent and large animal ovine models (Emmerson et al., 2019). In these studies, xenogeneic human eMSC delivered on a mesh in a model of subcutaneous wound repair, exerted a paracrine effect. DiO- or mCherry lentiviral-labeled eMSC implanted on a non-degradable polyamide/gelatin composite mesh, were detectable for 1–2 weeks in immunocompromised rats and mice (Ulrich et al., 2014a; Darzi et al., 2018) and up to 3 days in immune intact mice (Darzi et al., 2018). Despite this, the eMSC exerted marked paracrine effects, promoting early neovascularization, an anti-inflammatory response and supporting the deposition of physiological, crimped collagen rather than scar (Edwards et al., 2015). These changes induced by eMSC resulted in a clinically relevant outcome, a reduction in the stiffness of the mesh/tissue complex in the long term (90 days) (Ulrich et al., 2014a). In studies using degradable PLACL nanofiber/gelatin (Mukherjee et al., 2019b) or 3D printed PCL scaffolds (Paul et al., 2019), m-Cherry-labeled eMSC again reduced the foreign body response, slowed mesh degradation and induced endogenous cell influx into the scaffold in vivo, thereby promoting tissue repair. These are outcomes of clinically desired responses, particularly for application in pelvic organ prolapse repair surgery.
To more accurately model the translational approach to be used clinically, autologous, adventitial perivascular ovine eMSC (Table 2), labeled with paramagnetic nanoparticles conjugated to FITC were surgically delivered with non-degradable polyamide mesh in an ovine model of vaginal repair (Emmerson et al., 2019). A two-step procedure was required, firstly implanting the mesh followed by separate delivery of the eMSC in a collagen gel onto the mesh and crosslinking with blue light in situ, followed by suturing. Approximately 10–20% of the autologous eMSC survived 30 days following implantation. As observed in the rodent models, the eMSC modulated the inflammatory response and reduced myofibroblast accumulation around mesh filaments. Important lessons were learnt using this large animal model, particularly related to the separate delivery of eMSC and mesh. This modification to our protocol prevented one of the major adverse events associated with transvaginal mesh use, mesh exposure, that had resulted in the banning of polypropylene vaginal mesh by the FDA. This could not have been predicted from using mouse models of skin wound repair and shows the importance of closely recapitulating the clinical condition in large animal models before clinical translation of an MSC-based therapy.
Other Reparative Applications of eMSC
Human and monkey endometrial stromal fibroblasts transdifferentiated into cells with morphological, chemical and electrical activity of dopaminergic neurons have been investigated in several animal models. They engrafted and migrated to site of lesion in chemically induced mouse and green monkey (Table 2) models of Parkinson disease, differentiated to neuronal-like cells resulting in increased striatal dopamine and dopamine metabolite concentrations (Wolff et al., 2011, 2015). However, further studies on clinical outcomes such as the behavioral studies are warranted. Type 1 diabetes mellitus is a clinical condition which can benefit from islet-based cell transplantation. The plasticity of human endometrial stromal fibroblasts has been utilized to generate insulin secreting cells. These differentiated cells produced human insulin, decreased blood glucose levels and minimized diabetes-associated complications such as weight loss, dehydration, cataracts, delayed wound healing and sedative behavior in a mouse model of diabetes mellitus (Li et al., 2010; Santamaria et al., 2011). These differentiated cells were more efficient in a 3D construct, and were resistant to oxidative stress, normalized glucose levels and prolonged survival of the recipient mice (Li et al., 2010).
Both eMSC and endometrial fibroblasts show potential in regenerative and reparative medicine, with early studies suggesting capability in tissue engineering approaches using decellularized tissues or biomaterial scaffolds to deliver the cells to tissues requiring regeneration. Mode of action may be both cellular integration and paracrine.
MenSC in Regenerative or Reparative Medicine
MenSC are also an attractive cell source with potential for clinical application. MenSC have been assessed in various preclinical animal models of disease and in regenerative and reparative medicine (Table 5). Here, we will briefly review these studies.
Cardio-Reparative Potential
One of the first studies proposing the prospective use of MenSC differentiation in the clinical setting assessed the ability of MenSC to improve cardiac function after myocardial infarction. MenSC transplanted into a rat model ameliorated left ventricular systolic function and diminished fibrotic areas (Hida et al., 2008). In vivo differentiation of MenSC to cardiomyocytes in infarcted areas was demonstrated in the absence of recipient and donor cell fusion. Similar outcomes were obtained following MenSC injection into the ischemic zones of an immunocompetent rat model of myocardial infarction (Jiang et al., 2013). The therapy resulted in significant preservation of myocardial viability in the infarct zone and improvement in cardiac function, effects mostly attributed to the paracrine activity of MenSCs rather than transdifferentiation to myocardial cells.
Hepato-Reparative Potential
MenSC’s ability to differentiate toward hepatic cells in vitro (Khanjani et al., 2015) raised the possibility of MenSC application for treating liver disorders. Both MenSC and bmMSC, with the capacity to differentiate toward hepatocytes, prolonged the survival of mice with acute liver failure. MenSC localized in the damaged liver within 2 h following transplantation. This treatment also improved liver histology and architecture within weeks after MenSC injection. The MenSC therapy reduced serum levels of liver enzymes and metabolites (AST, ALT, urea, and total bilirubin) in the mice. Reduced hepatic degeneration, inflammatory cell infiltrate and collagen fiber deposition and improved glycogen storage were observed following MenSC administration (Fathi-Kazerooni et al., 2017), indicating the protective and reparative role of MenSC in acute liver damage.
It is likely that MenSC improvement of liver function is due to paracrine activity rather than differentiating into hepatocyte-like cells in improving liver fibrosis. In a murine model (Chen et al., 2017b), few MenSC differentiated into hepatocyte-like cells, suggesting the complexity of the in vivo milieu influencing MenSC differentiation. MenSC migrated to the fibrotic area, reduced already deposited collagen and significantly improved liver function. Nevertheless, end-stage liver fibrosis with few healthy hepatocytes may be a poor environment for survival of MenSC that also diminishes their ability to potentially differentiate into hepatocyte-like cells. This questions the utility of MenSC as a therapeutic modality to patients with cirrhosis. Further investigation in first reducing fibrosis in cirrhosis using MenSCs before attempting to regenerate hepatic tissue is warranted.
Ovarian Function and Reserve
The reparative capacities of MenSCs was exploited to treat premature ovarian failure (POF) in mouse/rat models (Liu et al., 2014; Feng et al., 2019; Manshadi et al., 2019; Yan et al., 2019). Intra-ovarian or intravenous injection of MenSCs reduced apoptosis and restored ovarian function, shown by expression of the ovarian markers, follicle-stimulating hormone receptor (Fshr), inhibin α/β, anti-Müllerian hormone (Amh), Ddx4 and Vegfa, and rising plasma levels of the ovarian hormones, estrogen and progesterone. Increased numbers of primary, mature and total ovarian follicle numbers were observed. DiI-labeled MenSC localized to GCs of immature ovarian follicles (Manshadi et al., 2019). Microarray analysis revealed greater similarity between mRNA expression patterns in the ovarian cells posttransplantation and human ovarian tissue than the pre-transplantation pattern in MenSCs, suggesting transdifferentiation into ovarian cells or fusion of MenSCs with murine cells (Liu et al., 2014). More importantly, mated mice grafted with MenSCs had more live births indicating the potential of MenSC to repair ovarian function (Feng et al., 2019). Mechanistic analyses revealed that mice treated with MenSCs improved ovarian microenvironment homeostasis through regulation of the ECM-dependent FAK/AKT signaling pathway (Feng et al., 2019). Similarly, in vitro, MenSC increased indices of mouse follicular growth, including survival rate, diameter and antrum formation, Bmp15 and Gdf9 expression and maturation rate in a 3D culture system. Secreted progesterone and estradiol increased in co-cultured murine preantral follicles and human MenSC implying a supportive role of MenSCs in follicle development, growth and maturation (Rajabi et al., 2018). In a recent clinical trial in 15 women, intraovarian injection of autologous MenSC in poor ovarian responders increased clinical pregnancy and live births (Zafardoust et al., 2020).
Collectively, MenSC impact on ovarian follicle development through paracrine signaling and potentially transdifferentiation highlights the possible utility of MenSC for ovarian function restoration in patients with POI and also fertility preservation approaches using in vitro follicle maturation. These pre-clinical findings and an early phase clinical trial warrant further investigation of the efficacy of MenSC for treatment of POI.
Lung Injury
MenSCs have been used to treat LPS-induced acute lung injury in a murine model (Xiang et al., 2017). Intravenously administered MenSC localized to the injured area and demonstrated repair of lung tissue morphology, improved lung microvascular permeability, and reduced clinical symptoms. MenSC exerted an anti-inflammatory effect, reducing lung inflammatory cells and IL-1β, whereas IL-10 was increased in both lung tissue and broncho-alveolar lavage fluid. Keratinocyte growth factor, which plays a major part in repair of damaged lung was increased, and together with decreased caspase-3 expression further supported the protective impact of the infused MenSC. It is likely that the reparative effect of MenSC can be ascribed to cell-cell contact and/or their paracrine function. Similarly, MenSC injection improved acute lung injury scores through normalization of lung O2 pressure. MenSC reduced neutrophil frequency, myeloperoxidase activity, and pro-inflammatory cytokines levels in bronchoalveolar fluid (Ren et al., 2018). MenSC injection also reduced inflammation and collagen deposition in a mouse model of bleomycin-induced pulmonary fibrosis (Zhao et al., 2018b). In summary, in acute lung injury and pulmonary fibrosis models, MenSC suppressed innate immune cells, lowered pro-inflammatory cytokines and increased IL-10 in lung, and lung function and morphology were restored, effects likely mediated by paracrine mechanisms.
Skin Wound Repair
The reparative potential of MenSCs in a murine excisional wound splinting model (Cuenca et al., 2018) showed that intradermal injection of MenSCs into a persistent wound improved healing and increased angiogenesis. Two pro-angiogenic genes, Il-8 and Vegf were upregulated in the wounds and there was maturation of wound vasculature. The MenSC-injected group also showed high density, and well-organized collagen fibers, suggesting MenSC have potential application for wound healing and cutaneous regeneration. MenSC seeded on an amniotic membrane improved wound closure in a rat excisional wound defect model (Farzamfar et al., 2018). In vitro, MenSCs differentiated into keratinocyte-like cells (Akhavan-Tavakoli et al., 2017), an effect potentiated in 3D culture (Fard et al., 2018) or in a biomimetic nanofibrous scaffold, two approaches that increased the wound healing capacity of MenSC (Arasteh et al., 2018). Such data imply the importance of ECM in the differentiation of MenSC into keratinocyte-like cells and further highlight the value of MenSCs as a potential therapeutic in clinical settings.
Intrauterine Adhesions and Endometrial Decidualization
The reparative potential of MenSC has been explored in a mouse model of endometrial injury and intrauterine adhesion (Zhang et al., 2016), where the endometrium is partially replaced by fibrotic scar tissue (Gargett and Ye, 2012). Intravascular injection of MenSCs rapidly repaired the injury by increasing microvessel density, resulting in increased endometrial thickness. Fertility was restored with a higher conception rate and larger numbers of implanted embryos compared to untreated controls. MenSC-conditioned medium promoted angiogenesis in vitro by activating Akt and Erk pathways and overexpression of genes involved in angiogenesis. Autologous cultured MenSCs administered to 7 women with severe Asherman’s syndrome, where intrauterine adhesions have replaced the endometrium, increased endometrial thickness to 7 mm in five of seven patients (Tan et al., 2016). Of the four patients who had undergone embryo transfer, two conceived and one had a spontaneous pregnancy, suggesting that MenSC reduced fibrosis and promoted functional endometrial repair of the injured endometrium.
Neuroprotective Potential
The application of MenSC, which can differentiate toward glia and neural cells in vitro (Table 3), for neuroprotection is an emerging research area. In an adult rat stroke model, intracerebral or intravenous MenSC transplantation ameliorated motor and behavioral symptoms and diminished neuronal cell death (Borlongan et al., 2010). Implantation of MenSC-seeded gelatin-based scaffolds in rats with sciatic nerve defect improved sciatic nerve function and reduced and gastrocnemius muscle loss to a similar level as bridging the nerve defect with autologous resected nerve segment (Farzamfar et al., 2017). MenSC also improved cognition function and memory defects in a mouse APP/PS1 model of Alzheimer’s disease (Zhao et al., 2018a). In the hippocampus, more activated microglia were observed which had altered function as less TNF and IL-1β were produced. These activated microglia had higher expression of insulin degrading enzyme and neprilysin, proteases responsible for Aβ plaque degradation. These results highlight the potential of MenSC in improvement of the pathological and cognitive defects in pre-clinical models of Alzheimer disease.
Other Potential Reparative Applications of MenSC
The regenerative ability of MenSC to promote muscle regeneration was demonstrated in a murine Mdx model of Duchenne muscular dystrophy, where skeletal muscles degenerate from lack of dystrophin. GFP-labeled MenSC were injected intramuscularly, where they fused with murine myoctes and restored dystrophin in the sarcolemma of muscle fibers (Cui et al., 2007). Similarly, MenSC fuse with myocytes in co-culture and subsequently express dystrophin.
MenSC can stimulate the regeneration of pancreatic islet β cells in a murine model (Wu et al., 2014). MenSC transplantation enhanced differentiation of endogenous endocrine progenitor cells into β-cells resulting in more normal islet morphology and structure, with improved hyperglycemia, glucose tolerance, insulin levels, body weight and survival rate. Although not investigated inflammation reduced by MenSC may have also contributed to the observed results.
In summary, MenSC show potential in regenerative medicine, with early studies suggesting capacity to influence tissue regeneration by paracrine mechanism, fusion and enhancement of endogenous tissue stem cell function.
Extracellular Vesicles as a Key Player of MenSC Reparative Function
Increasingly, the effects of MSC are attributed to their ability to secret extracellular vesicles (EVs), including exosomes (Murray and Krasnodembskaya, 2019). MenSCs also produce functionally active EVs that are homogeneous in size (30–170 nM) and express CD81, CD63, and TSG101 (Dalirfardouei et al., 2018). MenSC-EVs contain 895 proteins involved in complement activation, antigen processing and presentation, regulation of adaptive and innate immune responses, apoptosis control and signaling pathways (Marinaro et al., 2019). Licensing MenSCs with pro-inflammatory cytokines such as IFN-γ modulates EV protein cargo, increasing proteins involved in antigen processing and presentation, and miRNA content. MenSC-derived EVs also express high levels of ICAM-1, angiopoetin-2, angiogenin, osteoprotegerin, IL-6, and IL-8. In a mouse model of fulminant hepatic failure, pre-treatment with MenSC-exosomes showed higher survival rates, with reduced serum liver enzymes and pro-inflammatory cytokines, and well-organized hepatic structure (Chen et al., 2017a). Hepatocyte apoptosis and proliferation of liver macrophages were reduced. In a rat model of diabetes, MenSC-derived EVs were tracked in, and enhanced the number of pancreatic β cell islets and raised serum insulin, without impact on non-fasting blood glucose (Mahdipour et al., 2019). MenSC-derived exosomes impacted wound healing in a mouse model of diabetes through M2 macrophage polarization, induction of neoangiogenesis and re-epithelialization (Dalirfardouei et al., 2019).
In summary, the beneficial impacts of MenSC treatment are mostly attributed to the anti-inflammatory properties of MenSC-derived EVs. In some settings the modulatory effects of EVs were superior to the use of intact MenSC. Therapeutic utilization of MenSC-EVs might be advantageous over whole cells by improving the therapeutic index, by introduction of less protein, avoidance of allo- and xenogeneic reactions and their off-the-shelf potential. Nonetheless, their nano-scale size results in rapid clearance from the body necessitating repeated administration in large quantities, although therapy targeted to damaged tissue may overcome this potential challenge. Taken together, MenSC may qualify as a promising therapeutic cell type for future clinical applications. Nevertheless, as with any other treatment modality, the safety of MenSC in the clinical settings needs addressing.
Clinical Translation of eMSC and MenSC
There are hundreds of clinical trials using MSC either in progress or completed and thousands of patients have been treated. Apart from several spectacular successes the overall results of these clinical trials have been underwhelming (Prockop et al., 2014). MSC were rushed to the clinic before many issues were resolved, including their mechanism of action and the heterogeneity of MSC products. Heterogeneity arises from variability in isolation methods and during culture expansion (Prockop et al., 2014). Thawing cryostored MSC induces cell injury lasting up to 24 h (Moll et al., 2016), suggesting a lack of fitness for purpose of culture-expanded, thawed MSC (Galipeau and Sensebe, 2018). There is also a need for potency assays based on MSC mechanism of action in vivo (Prockop et al., 2014; Galipeau and Sensebe, 2018). These are important considerations for investigators seeking to translate eMSC and MenSC as cell-based therapies.
Serum Free Culture Protocols
The clinical translation of human MSC depends upon largescale production of a homogeneous cell population that is safe and reproducible. Factors contributing to a heterogeneous population are isolation protocol, culture medium and culture environment. The discovery of a single surface marker for human eMSC, SUSD2, has enabled relatively easy purification of a homogeneous starting population. Choice of culture medium and environment majorly influence the generation of large scale homogeneous eMSC and MSC from other sources utilized for clinical use. Until recently, MSC including eMSC have been cultured in fetal calf serum (FCS) containing medium in 20% O2 environment (Schwab and Gargett, 2007). FCS provides extracellular matrix, growth factors, hormones and many other nutrients promoting cell grow and proliferation (Cantor, 2019). However, FCS is not a defined source of nutrients with batch to batch variation and presence of unknown components that contribute to heterogeneity (Shahdadfar et al., 2005). Some FCS components induce differentiation, reducing the population of potent MSC (Shahdadfar et al., 2005; Labome, 2012). The use of FCS for in vitro MSC expansion also carries a rare risk of xeno-immunization and zoonotic transmission (Hawkes, 2015). Defined medium devoid of FCS is required to produce homogeneous, undifferentiated potent MSC using good manufacturing practices (GMP) for safe use in humans. Replacing FCS with human products including human serum, platelet-poor or -rich plasma and platelet lysate have been used (Dessels et al., 2016). Although more physiological, these alternatives have similar composition variability as FCS and may also induce differentiation, resulting in inconsistent, non-reproducible cell products. Gene profiling of primary CD140b+CD146+ eMSC showed distinct differences to endometrial stromal fibroblasts (CD140b+CD146 –) (Spitzer et al., 2012) and extensive eMSC cultivation in FCS medium in 20% O2 led down-regulation of 81% of eMSC-related genes and up-regulation of 55% of fibroblast-associated genes, verifying culture-induced spontaneous differentiation and reduced functionality (Barragan et al., 2016; Gargett and Gurung, 2016). A defined medium and physiological O2 environment that generates homogeneous undifferentiated eMSC which are efficacious, safe and reproducible under cGMP guidelines would be appropriate for clinical applications such as pelvic organ prolapse and Asherman’s syndrome.
While various serum-free media are available for culturing human MSC, vast research shows that “one size does not fit all,” indicting the need to identify the specific niche environment for eMSC. Indeed, eMSC attachment and growth were best supported by a fibronectin matrix in xeno-free DMEM supplemented with FGF2 and EGF (SFM) in 5% O2, compared to other serum-free commercial media, Lonza-TP-SF and Stem Pro-XF giving similar growth rates to serum-containing medium (Rajaraman et al., 2013). Despite this, like all MSC, the expanded SUSD2+ eMSC spontaneously differentiated to non-clonogenic stromal fibroblasts with loss of the eMSC surface markers SUSD2, CD140b, and CD146 indicating cellular heterogeneity and decreased potency (Gurung et al., 2018b).
Small Molecules to Maintain Undifferentiated eMSC State
Understanding the intrinsic signaling pathways involved in stem-cell fate, self-renewal, proliferation and differentiation, and manipulating them using chemical approaches enables generation of homogeneous potent cells for cell-based therapies (Xu et al., 2008; Li and Ding, 2010). Chemically defined media needs optimization for each cell type using relative cell-growth as a guide. Augmenting SFM with a small molecule (A83-01) targeting the transforming growth factor-β1 receptor (TGFβR) signaling pathway involved in cellular differentiation and enhanced cell growth, mitigated loss of undifferentiated, clonogenic eMSC, overcoming this major bottleneck for clinical translation of eMSC (Gurung et al., 2015, 2018b). A83-01 maintained ISCT properties of eMSC, promoted proliferation of homogeneous SUSD2+ eMSC and prevented apoptosis and senescence. Correlation between SUSD2 expression and TGFβ-induced senescence and cell death was also demonstrated in cancer cells using small interfering RNA and TGFβ (Zhang et al., 2017). The potency of A83-01-treated eMSC was further validated by transcriptome profiling, providing insight into the biological nature of eMSC and the probable therapeutic mode of action (Gurung et al., 2015, 2018b). The complexity of TGFβR signaling in eMSC was defined through identification of ∼1200 differentially regulated genes by A83-01 involved in anti-inflammatory responses, angiogenesis, cell migration and proliferation, collagen fibril and extracellular matrix organization, anti-fibrosis and anti-apoptosis (Gurung et al., 2018b). The potency of A83-01-treated eMSC was established by demonstrating increased expression of recently described bmMSC potency genes; TWIST1, TWIST2, JAG1, LIFR, and SLIT2 (Samsonraj et al., 2015; Gurung et al., 2018b). These and our previous findings also highlighted the need for additional surface markers to identify potent MSC and for specific tissues (Rajaraman et al., 2013; Gurung et al., 2015, 2018b; Samsonraj et al., 2015).
The reparative and regenerative capacity of MSC is mainly driven by the secretome or EVs that promote angiogenesis, ECM regulation, immunoregulation and antimicrobial activity. Transcriptional profiling of A83-01-treated eMSC revealed important cellular secretome-regulating genes involved in angiogenesis, anti-fibrosis and immunomodulation; HGF, VCAM1, PGF, HPSE, SOD3, SOD2, and SOD1. Secretome analysis of the conditioned medium supported transcriptional findings with increased HGF, PTX3, CCL2, IGFBP-2, CCL2, GM-CSF, IGFBP-2, DKK-1, and EGF and decreased THBS1, EMMPRIN, and OPN. HLA-G, a nonclassical MHC-I molecule, and immunomodulatory cytokine functioning at the maternal-fetal interface and some immune privileged adult tissues, was ubiquitously expressed in A83-01-treated eMSC, together with HLA-A-C and HLA-E and F, but not HLA-II, an indicator of good tolerance for allogeneic transplantation. HLA-G has been identified in several MSC types (Selmani et al., 2008; Yang et al., 2012; Naji et al., 2013; Ding et al., 2016), and together with other MHC-I molecules correlates directly to their immunosuppressive effect (Yang et al., 2012). These molecules are surrogate prognostic factors used to monitor disease progression or efficacy of transplantation (Nasef et al., 2007). Likewise, RNA-sequencing revealed the first evidence of the ability of A83-01-treated eMSC to secrete exosomes (Gurung et al., 2018b), that may function in their paracrine action. Further transcriptome analysis showed evidence of the antimicrobial potential of A83-01-treated eMSC partially through the secretion of antimicrobial peptides (AMPs), chromogranin B, liver enriched AMP-2 and secretogranin II (Gurung et al., 2018b). Taken together, these findings signify the clinical potential of eMSC and open avenues to examine their use in regenerative medicine as an “off the shelf” as well as cell-derived therapy.
Safety of eMSC
Spontaneous transformation of MSC is a potential concern for therapeutics, although thorough investigations of earlier studies showed cellular-cross contamination. To date, no cancer due to primary-culture expanded MSC, has been diagnosed in human clinical trials. Nonetheless, the risk of such potential events should be investigated by utilizing surrogate assays, such as telomere length, pluripotency genes, tumor formation, genomic instability, karyotyping and DNA damage response through transcriptomics and in vitro and in vivo assays. A83-01-expanded eMSC promote telomere stability through TERC (Telomerase RNA Component), TERF1 and 2 (Telomeric Repeat Binding Factors 1 and 2), TINF2 (TERF1 Interacting Nuclear Factor 2), TERF2IP (TERF2 Interacting Protein), TNKS (Tankyrase) and POT1 (Protection of Telomere 1) which collaborate to regulate telomere length and protect cells from chromosomal damage (Gurung et al., 2018b). Using our isolation and expansion protocol, SUSD2+ eMSC do not express pluripotency-associated genes, unlike hESC and iPSCs with unlimited growth and tumorigenic potential (Gurung et al., 2015; Gurung et al., 2018b). Although SFM/5%O2/A83-01 culture-expanded eMSC survived longer in vivo than culture in FCS/20%O2 medium, their lifespan was limited and tumors were not detected (Gurung et al., 2018a). Considerable progress has been made in developing expansion protocols for perivascular eMSC showing promise in their safety profile.
Safety of MenSC
Donor Age
Age of donor affects many of MSC functions; cytokine secretion, paracrine activity, anti-apoptotic mechanisms, hematopoietic stem cell supporting capacity and proliferation rate, all of which reduce with aging (Alt et al., 2012). However, the proliferative capacity of MenSC does not appear donor-dependent in individuals aged up to 40 years, but MenSC from older donors had a weaker potential for long-term passaging (Chen et al., 2015).
Passage Number
Menstrual blood can be processed up to 72 h after collection without significant change in properties of the plastic adherent MenSC (Liu et al., 2018). As mentioned, culture expansion of MSC is required to obtain enough cells for preclinical or clinical applications. The proliferation rate of MenSC gradually decreased with increasing passage number (Chen et al., 2015), with highly passaged cells increasing in size, losing fibroblastic morphology and appearing senescent. Important signaling molecules, including MAPK, molecules involved in carcinogenesis (PPAR and P53) and immune responses changed with MenSC passage number. Passaging induces aging of MenSC and alters genes involved in transcriptional regulation, stress response, cell proliferation, development and apoptosis, and karyotype at high passages (P20). MenSC underwent aging after 45 PD (Zemel’ko et al., 2011), but even after 68 PDs of a single MenSC sample, maintained a normal karyotype and did not develop tumors (Meng et al., 2007). Multiplex ligation-dependent probe amplification of MenSC genomic DNA at passages two and twelve showed that MenSC maintained a diploid phenotype without chromosomal aberrations (Khanmohammadi et al., 2014), consistent with other reports of a normal female karyotype without chromosomal aberrations using standard cytogenetics at passage 12 (Cui et al., 2007; Patel et al., 2008). Pluripotency protein expression reduced during passaging of MenSCs, from 97.5% OCT-4+ cells at the first passage to 19.4% at passage twelve (Khanmohammadi et al., 2014), important for clinical translation. These observations warrant standardization of MenSC isolation and culture protocols for clinical applications.
Safety of MenSC
The risk of tumorigenicity, toxicity, autoimmunity, and/or endometriosis following MenSCs injection are crucial safety issues to be established before using MenSC in the clinic. Angiogenic activity of MenSC, raises concern of promoting tumor progression. In a C6 model of rat glioma MenSCs not only failed to stimulate, but also inhibited tumor growth (Han et al., 2009). MenSCs showed no tumor formation in a xenograft rat model of brain stroke with no concurrent immunosuppression (Borlongan et al., 2010). Several acute and chronic tumorigenicity/toxicity experiments in immunocompromised mice using MenSC in clinically relevant doses showed no toxic effects on body and organ weight, biochemistry following necropsy or histopathological changes at injection sites (Bockeria et al., 2013). Concerns of ectopic bone formation on clinical application of bmMSC, are mitigated by the weak osteogenic differentiation capacity of MenSC and could be advantageous for clinical translation.
The next step prior to clinical development of MenSC is to undertake cell tracking of reliably labeled MenSC in vivo and determining their mechanism of action. Also important is the development of relevant large animal pre-clinical models of the targeted disease for evaluating MenSC, to improve the likelihood of translating rodent studies into clinical practice. In 2008, four multiple sclerosis patients were injected both intrathecally and intravenously with in vitro expanded allogeneic MenSC. Functional and radiological evaluations demonstrated no disease status progression and none of the treated patients showed allergic reactions or ectopic tissue formation (Zhong et al., 2009). No adverse effects were observed in the patients 4 years later in the last reported follow up (Bockeria et al., 2013). Clearly more investigation is required to determine the safety of MenSC for clinical translation.
Identification of eMSC potency makers and serum-free expansion media have enabled gene sequencing of the primary cells and their expanded progeny (Spitzer et al., 2012; Barragan et al., 2016; Gurung et al., 2018b). Comprehensive RNA-seq analysis of SFM/5%O2/A83-01-treated eMSC described the gene expression profiles and uncovered vast range of regulatory pathways which not only aided in understating their mechanism of action but also suggested their potential for broader applications. Much progress are made in understanding the in vitro and in vivo roles of eMSC, setting a strong foundation for clinical application. Further research to understand and validate eMSC’s potential through careful titration of assays and selection of animal models relative to their potential use is vital.
Summary
eMSC and MenSC have fulfilled the ISCT criteria for MSC, but thus far, no comparative study has been performed to delineate the potential link between these two MSC types. Nonetheless, considering that eMSC comprise a small cell population residing around endometrial blood vessels while MenSC represent majority of endometrial stromal cells, it seems that the latter is more heterogeneous. Although multi-differentiation capacity of eMSC and MenSC has been documented in vitro, the reparative properties of these cells in vivo seem to stem from their paracrine rather than differentiation capacity and integration into tissue. Akin to MSC from other sources, MenSC and eMSC have profound immunomodulatory effects and attenuate inflammation, although there are distinct differences highlighting their unique tissue of origin. eMSC and MenSC have been evaluated in numerous preclinical animal models of disease showing substantial angiogenic, anti-fibrotic and anti-inflammatory effects although it is too early to draw a firm conclusion on the same impacts in clinical settings. Culture protocols have been developed that maintain perivascular eMSC in the undifferentiated state during culture expansion, which now need testing in animal models to determine if this more homogeneous MSC product further improves in vivo angiogenesis, antifibrosis, anti-inflammatory and prohealing effects. Both eMSC, easily procured without anesthetic, and non-invasively obtained MenSC, imbued with both reparative and immunomodulatory properties are likely promising therapeutic cell types for future clinical applications. Nevertheless, multiple variables need to be standardized particularly heterogeneity of MenSC and the safety and therapeutic potency of eMSC and MenSC in clinical settings needs to be extensively addressed.
Author Contributions
CG and A-HZ contributed to the conception and review design and revised the manuscript. MB, SG, SD, SN, SK, A-HZ, and CG wrote sections of the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the National Health and Medical Research Council (NHMRC) of Australia Project Grants (ID 1159677 to CG) and Investigator Grant (ID 1173882 to CG) and the Victorian Government’s Operational Infrastructure Support Program, and Avicenna Research Institute (ARI) Project Grants (ID 960110-029 to SK).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We are sincerely thankful to Dr. Maryam Tavakoli and Samira Hosseini for their assistance in providing related manuscripts and to Zahra Saffarian for office work.
References
Akhavan-Tavakoli, M., Fard, M., Khanjani, S., Zare, S., Edalatkhah, H., Mehrabani, D., et al. (2017). In vitro differentiation of menstrual blood stem cells into keratinocytes: a potential approach for management of wound healing. Biologicals 48, 66–73. doi: 10.1016/j.biologicals.2017.05.005
Alcayaga-Miranda, F., Cuenca, J., Luz-Crawford, P., Aguila-Diaz, C., Fernandez, A., Figueroa, F. E., et al. (2015a). Characterization of menstrual stem cells: angiogenic effect, migration and hematopoietic stem cell support in comparison with bone marrow mesenchymal stem cells. Stem Cell Res. Ther. 6, 32.
Alcayaga-Miranda, F., Cuenca, J., Martin, A., Contreras, L., Figueroa, F. E., and Khoury, M. (2015b). Combination therapy of menstrual derived mesenchymal stem cells and antibiotics ameliorates survival in sepsis. Stem Cell Res. Ther. 6:199.
Aleahmad, M., Ghanavatinejad, A., Bozorgmehr, M., Shokri, M. R., Nikoo, S., Tavakoli, M., et al. (2018). Menstrual Blood-Derived Stromal Stem Cells Augment CD4+ T Cells Proliferation. Avicenna J. Med. Biotechnol. 10, 183–191.
Allavena, P., Piemonti, L., Longoni, D., Bernasconi, S., Stoppacciaro, A., Ruco, L., et al. (1998). IL-10 prevents the differentiation of monocytes to dendritic cells but promotes their maturation to macrophages. Eur. J. Immunol. 28, 359–369. doi: 10.1002/(sici)1521-4141(199801)28:01<359::aid-immu359>3.0.co;2-4
Allickson, J. G., Sanchez, A. M., Yefimenko, N., Borlongan, C. V., and Sanberg, P. R. (2011). Recent studies assessing the proliferative capability of a novel adult stem cell identified in menstrual blood. Open Stem Cell J. 3:4. doi: 10.2174/1876893801103010004
Alt, E. U., Senst, C., Murthy, S. N., Slakey, D. P., Dupin, C. L., Chaffin, A. E., et al. (2012). Aging alters tissue resident mesenchymal stem cell properties. Stem Cell Res. 8, 215–225. doi: 10.1016/j.scr.2011.11.002
Arasteh, S., Katebifar, S., Shirazi, R., and Kazemnejad, S. (2018). Differentiation of Menstrual Blood Stem Cells into Keratinocyte-Like Cells on Bilayer Nanofibrous Scaffold. Methods Mol. Biol. 2125, 129–156. doi: 10.1007/7651_2018_193
Augello, A., Tasso, R., Negrini, S. M., Amateis, A., Indiveri, F., Cancedda, R., et al. (2005). Bone marrow mesenchymal progenitor cells inhibit lymphocyte proliferation by activation of the programmed death 1 pathway. Eur. J. Immunol. 35, 1482–1490. doi: 10.1002/eji.200425405
Azedi, F., Kazemnejad, S., Zarnani, A. H., Behzadi, G., Vasei, M., Khanmohammadi, M., et al. (2014). Differentiation potential of menstrual blood- versus bone marrow-stem cells into glial-like cells. Cell Biol. Int. 38, 615–624. doi: 10.1002/cbin.10245
Azedi, F., Kazemnejad, S., Zarnani, A. H., Soleimani, M., Shojaei, A., and Arasteh, S. (2017). Comparative capability of menstrual blood versus bone marrow derived stem cells in neural differentiation. Mol. Biol. Rep. 44, 169–182. doi: 10.1007/s11033-016-4095-7
Barragan, F., Irwin, J. C., Balayan, S., Erikson, D. W., Chen, J. C., Houshdaran, S., et al. (2016). Human Endometrial Fibroblasts Derived from Mesenchymal Progenitors Inherit Progesterone Resistance and Acquire an Inflammatory Phenotype in the Endometrial Niche in Endometriosis. Biol. Reprod. 94:118.
Bartholomew, A., Sturgeon, C., Siatskas, M., Ferrer, K., McIntosh, K., Patil, S., et al. (2002). Mesenchymal stem cells suppress lymphocyte proliferation in vitro and prolong skin graft survival in vivo. Exp. Hematol. 30, 42–48. doi: 10.1016/s0301-472x(01)00769-x
Bianco, P., Cao, X., Frenette, P. S., Mao, J. J., Robey, P. G., Simmons, P. J., et al. (2013). The meaning, the sense and the significance: translating the science of mesenchymal stem cells into medicine. Nat. Med. 19, 35–42. doi: 10.1038/nm.3028
Bockeria, L., Bogin, V., Bockeria, O., Le, T., Alekyan, B., Woods, E. J., et al. (2013). Endometrial regenerative cells for treatment of heart failure: a new stem cell enters the clinic. J. Transl. Med. 11, 56. doi: 10.1186/1479-5876-11-56
Bodek, G., Bukowska, J., Wisniewska, J., and Ziecik, A. J. (2015). Evidence for the presence of stem/progenitor cells in porcine endometrium. Mol. Reprod. Dev. 82, 182–190. doi: 10.1002/mrd.22459
Borlongan, C. V., Kaneko, Y., Maki, M., Yu, S. J., Ali, M., Allickson, J. G., et al. (2010). Menstrual blood cells display stem cell-like phenotypic markers and exert neuroprotection following transplantation in experimental stroke. Stem Cells Dev. 19, 439–452. doi: 10.1089/scd.2009.0340
Bourin, P., Bunnell, B. A., Casteilla, L., Dominici, M., Katz, A. J., March, K. L., et al. (2013). Stromal cells from the adipose tissue-derived stromal vascular fraction and culture expanded adipose tissue-derived stromal/stem cells: a joint statement of the International Federation for Adipose Therapeutics and Science (IFATS) and the International Society for Cellular Therapy (ISCT). Cytotherapy 15, 641–648. doi: 10.1016/j.jcyt.2013.02.006
Bozorgmehr, M., Moazzeni, S. M., Salehnia, M., Sheikhian, A., Nikoo, S., and Zarnani, A. H. (2014). Menstrual blood-derived stromal stem cells inhibit optimal generation and maturation of human monocyte-derived dendritic cells. Immunol. Lett. 162, 239–246. doi: 10.1016/j.imlet.2014.10.005
Bukowska, J., Ziecik, A. J., Laguna, J., Gawronska-Kozak, B., and Bodek, G. (2015). The Importance of the Canonical Wnt signaling pathway in the porcine endometrial stromal stem/progenitor cells: implications for regeneration. Stem Cells Dev. 24, 2873–2885. doi: 10.1089/scd.2015.0078
Busser, H., Najar, M., Raicevic, G., Pieters, K., Velez Pombo, R., Philippart, P., et al. (2015). Isolation and Characterization of Human Mesenchymal stromal cell subpopulations: comparison of bone marrow and adipose tissue. Stem Cells Dev. 24, 2142–2157. doi: 10.1089/scd.2015.0172
Cabezas, J., Lara, E., Pacha, P., Rojas, D., Veraguas, D., Saravia, F., et al. (2014). The endometrium of cycling cows contains populations of putative mesenchymal progenitor cells. Reprod. Domest. Anim. 49, 550–559. doi: 10.1111/rda.12309
Cabezas, J., Rojas, D., Navarrete, F., Ortiz, R., Rivera, G., Saravia, F., et al. (2018). Equine mesenchymal stem cells derived from endometrial or adipose tissue share significant biological properties, but have distinctive pattern of surface markers and migration. Theriogenology 106, 93–102. doi: 10.1016/j.theriogenology.2017.09.035
Campo, H., Baptista, P. M., Lopez-Perez, N., Faus, A., Cervello, I., and Simon, C. (2017). De- and recellularization of the pig uterus: a bioengineering pilot study. Biol. Reprod. 96, 34–45. doi: 10.1095/biolre/bio143396
Cantor, J. R. (2019). The rise of physiologic media. Trends Cell Biol. 29, 854–861. doi: 10.1016/j.tcb.2019.08.009
Caplan, A. I. (2016). MSCs: the sentinel and safe-guards of injury. J. Cell. Physiol. 231, 1413–1416. doi: 10.1002/jcp.25255
Cervello, I., Martinez-Conejero, J. A., Horcajadas, J. A., Pellicer, A., and Simon, C. (2007). Identification, characterization and co-localization of label-retaining cell population in mouse endometrium with typical undifferentiated markers. Hum. Reprod. 22, 45–51. doi: 10.1093/humrep/del332
Chaminadas, G., Propper, A. Y., Royez, M., Prost, O., Remy-Martin, J. P., and Adessi, G. L. (1986). Culture of epithelial and stromal cells of guinea-pig endometrium and the effect of oestradiol-17 beta on the epithelial cells. J. Reprod. Fertil. 77, 547–558. doi: 10.1530/jrf.0.0770547
Chan, R. W., and Gargett, C. E. (2006). Identification of label-retaining cells in mouse endometrium. Stem Cells 24, 1529–1538. doi: 10.1634/stemcells.2005-0411
Chan, R. W., Kaitu’u-Lino, T., and Gargett, C. E. (2012). Role of label-retaining cells in estrogen-induced endometrial regeneration. Reprod. Sci. 19, 102–114. doi: 10.1177/1933719111414207
Chan, R. W., Schwab, K. E., and Gargett, C. E. (2004). Clonogenicity of human endometrial epithelial and stromal cells. Biol. Reprod. 70, 1738–1750. doi: 10.1095/biolreprod.103.024109
Chen, J., Du, X., Chen, Q., and Xiang, C. (2015). Effects of donors’ age and passage number on the biological characteristics of menstrual blood-derived stem cells. Int. J. Clin. Exp. Pathol. 8:14584.
Chen, L., Xiang, B., Wang, X., and Xiang, C. (2017a). Exosomes derived from human menstrual blood-derived stem cells alleviate fulminant hepatic failure. Stem Cell Res. Ther. 8:9.
Chen, L., Zhang, C., Chen, L., Wang, X., Xiang, B., Wu, X., et al. (2017b). Human Menstrual Blood-Derived Stem Cells Ameliorate liver fibrosis in mice by targeting hepatic stellate cells via paracrine mediators. Stem Cells Transl. Med. 6, 272–284. doi: 10.5966/sctm.2015-0265
Chomarat, P., Banchereau, J., Davoust, J., and Palucka, A. K. (2000). IL-6 switches the differentiation of monocytes from dendritic cells to macrophages. Nat. Immunol. 1, 510–514. doi: 10.1038/82763
Corcione, A., Benvenuto, F., Ferretti, E., Giunti, D., Cappiello, V., Cazzanti, F., et al. (2006). Human mesenchymal stem cells modulate B-cell functions. Blood 107, 367–372.
Corselli, M., Chen, C. W., Sun, B., Yap, S., Rubin, J. P., and Peault, B. (2012). The tunica adventitia of human arteries and veins as a source of mesenchymal stem cells. Stem Cells Dev. 21, 1299–1308. doi: 10.1089/scd.2011.0200
Couri, B. M., Lenis, A. T., Borazjani, A., Paraiso, M. F., and Damaser, M. S. (2012). Animal models of female pelvic organ prolapse: lessons learned. Expert Rev. Obstet. Gynecol. 7, 249–260. doi: 10.1586/eog.12.24
Crisan, M., Corselli, M., Chen, W. C., and Peault, B. (2012). Perivascular cells for regenerative medicine. J. Cell Mol. Med. 16, 2851–2860. doi: 10.1111/j.1582-4934.2012.01617.x
Crisan, M., Yap, S., Casteilla, L., Chen, C. W., Corselli, M., Park, T. S., et al. (2008). A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell 3, 301–313. doi: 10.1016/j.stem.2008.07.003
Cuenca, J., Le-Gatt, A., Castillo, V., Belletti, J., Diaz, M., Kurte, M., et al. (2018). The reparative abilities of menstrual stem cells modulate the wound matrix signals and improve cutaneous regeneration. Front. Physiol. 9:464. doi: 10.3389/fphys.2018.00464
Cui, C. H., Uyama, T., Miyado, K., Terai, M., Kyo, S., Kiyono, T., et al. (2007). Menstrual blood-derived cells confer human dystrophin expression in the murine model of Duchenne muscular dystrophy via cell fusion and myogenic transdifferentiation. Mol. Biol. Cell 18, 1586–1594. doi: 10.1091/mbc.e06-09-0872
da Silva Meirelles, L., Chagastelles, P. C., and Nardi, N. B. (2006). Mesenchymal stem cells reside in virtually all post-natal organs and tissues. J. Cell Sci. 119, 2204–2213. doi: 10.1242/jcs.02932
Dalal, J., Gandy, K., and Domen, J. (2012). Role of mesenchymal stem cell therapy in Crohn’s disease. Pediatr. Res. 71, 445–451.
Dalirfardouei, R., Jamialahmadi, K., Jafarian, A. H., and Mahdipour, E. (2019). Promising effects of exosomes isolated from menstrual blood-derived mesenchymal stem cell on wound-healing process in diabetic mouse model. J. Tissue Eng. Regen. Med. 13, 555–568. doi: 10.1002/term.2799
Dalirfardouei, R., Jamialahmadi, K., and Mahdipour, E. (2018). A feasible method for the isolation of mesenchymal stem cells from menstrual blood and their exosomes. Tissue Cell 55, 53–62. doi: 10.1016/j.tice.2018.09.010
Darzi, S., Deane, J. A., Nold, C. A., Edwards, S. E., Gough, D. J., Mukherjee, S., et al. (2018). Endometrial Mesenchymal Stem/Stromal Cells Modulate the Macrophage Response to Implanted Polyamide/Gelatin Composite Mesh in Immunocompromised and Immunocompetent Mice. Sci. Rep. 8:6554.
Darzi, S., Werkmeister, J. A., Deane, J. A., and Gargett, C. E. (2016). Identification and characterization of human endometrial mesenchymal stem/stromal cells and their potential for cellular therapy. Stem Cells Transl. Med. 5, 1127–1132. doi: 10.5966/sctm.2015-0190
Darzi, S., Zarnani, A. H., Jeddi-Tehrani, M., Entezami, K., Mirzadegan, E., Akhondi, M. M., et al. (2012). Osteogenic differentiation of stem cells derived from menstrual blood versus bone marrow in the presence of human platelet releasate. Tissue Eng. Part A 18, 1720–1728. doi: 10.1089/ten.tea.2011.0386
de Carvalho Rodrigues, D., Asensi, K. D., Vairo, L., Azevedo-Pereira, R. L., Silva, R., Rondinelli, E., et al. (2012). Human menstrual blood-derived mesenchymal cells as a cell source of rapid and efficient nuclear reprogramming. Cell Transplant. 21, 2215–2224. doi: 10.3727/096368912x653048
Dessels, C., Potgieter, M., and Pepper, M. S. (2016). Making the switch: alternatives to fetal bovine serum for adipose-derived stromal cell expansion. Front. Cell Dev. Biol. 4:115. doi: 10.3389/fcell.2016.00115
Di Nicola, M., Carlo-Stella, C., Magni, M., Milanesi, M., Longoni, P. D., Matteucci, P., et al. (2002). Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood 99, 3838–3843. doi: 10.1182/blood.v99.10.3838
Dimmeler, S., Ding, S., Rando, T. A., and Trounson, A. (2014). Translational strategies and challenges in regenerative medicine. Nat. Med. 20, 814–821. doi: 10.1038/nm.3627
Ding, D. C., Chou, H. L., Chang, Y. H., Hung, W. T., Liu, H. W., and Chu, T. Y. (2016). Characterization of HLA-G and related immunosuppressive effects in human umbilical cord stroma-derived stem cells. Cell Transplant. 25, 217–228. doi: 10.3727/096368915x688182
Dominici, M., Le Blanc, K., Mueller, I., Slaper-Cortenbach, I., Marini, F., Krause, D., et al. (2006). Minimal criteria for defining multipotent mesenchymal stromal cells. the international society for cellular therapy position statement. Cytotherapy 8, 315–317. doi: 10.1080/14653240600855905
Domnina, A. P., Novikova, P. V., Fridlyanskaya, I. I., Shilina, M. A., Zenin, V. V., and Nikolsky, N. N. (2016). Induction of decidual differentiation in endometrial mesenchymal stem cells. Cell Tissue Biol. 10, 95–99. doi: 10.1134/s1990519x16020048
Dosiou, C., and Giudice, L. C. (2005). Natural killer cells in pregnancy and recurrent pregnancy loss: endocrine and immunologic perspectives. Endocr. Rev. 26, 44–62. doi: 10.1210/er.2003-0021
Ebrahimi-Barough, S., Kouchesfahani, H. M., Ai, J., and Massumi, M. (2013). Differentiation of human endometrial stromal cells into oligodendrocyte progenitor cells (OPCs). J. Mol. Neurosci. 51, 265–273. doi: 10.1007/s12031-013-9957-z
Edwards, S. L., Ulrich, D., White, J. F., Su, K., Rosamilia, A., Ramshaw, J. A., et al. (2015). Temporal changes in the biomechanical properties of endometrial mesenchymal stem cell seeded scaffolds in a rat model. Acta Biomater. 13, 286–294. doi: 10.1016/j.actbio.2014.10.043
Emmerson, S., Mukherjee, S., Melendez-Munoz, J., Cousins, F., Edwards, S. L., Karjalainen, P., et al. (2019). Composite mesh design for delivery of autologous mesenchymal stem cells influences mesh integration, exposure and biocompatibility in an ovine model of pelvic organ prolapse. Biomaterials 225:119495. doi: 10.1016/j.biomaterials.2019.119495
Emmerson, S. J., and Gargett, C. E. (2016). Endometrial mesenchymal stem cells as a cell based therapy for pelvic organ prolapse. World J. Stem Cells 8, 202–215.
Faramarzi, H., Mehrabani, D., Fard, M., Akhavan, M., Zare, S., Bakhshalizadeh, S., et al. (2016). The potential of menstrual blood-derived stem cells in differentiation to epidermal lineage: A preliminary report. World J. Plastic Surg. 5:26.
Fard, M., Akhavan-Tavakoli, M., Khanjani, S., Zare, S., Edalatkhah, H., Arasteh, S., et al. (2018). Bilayer amniotic membrane/nano-fibrous fibroin scaffold promotes differentiation capability of menstrual blood stem cells into keratinocyte-like cells. Mol. Biotechnol. 60, 100–110. doi: 10.1007/s12033-017-0049-0
Farzamfar, S., Naseri-Nosar, M., Ghanavatinejad, A., Vaez, A., Zarnani, A. H., and Salehi, M. (2017). Sciatic nerve regeneration by transplantation of menstrual blood-derived stem cells. Mol. Biol. Rep. 44, 407–412. doi: 10.1007/s11033-017-4124-1
Farzamfar, S., Salehi, M., Ehterami, A., Naseri-Nosar, M., Vaez, A., Zarnani, A. H., et al. (2018). Promotion of excisional wound repair by a menstrual blood-derived stem cell-seeded decellularized human amniotic membrane. Biomed. Eng. Lett. 8, 393–398. doi: 10.1007/s13534-018-0084-1
Fathi-Kazerooni, M., Tavoosidana, G., Taghizadeh-Jahed, M., Khanjani, S., Golshahi, H., Gargett, C. E., et al. (2017). Comparative restoration of acute liver failure by menstrual blood stem cells compared with bone marrow stem cells in mice model. Cytotherapy 19, 1474–1490. doi: 10.1016/j.jcyt.2017.08.022
Feng, P., Li, P., and Tan, J. (2019). Human menstrual blood-derived stromal cells promote recovery of premature ovarian insufficiency via regulating the ECM-dependent FAK/AKT signaling. Stem Cell Rev. Rep. 15, 241–255. doi: 10.1007/s12015-018-9867-0
Frankman, E. A., Alperin, M., Sutkin, G., Meyn, L., and Zyczynski, H. M. (2013). Mesh exposure and associated risk factors in women undergoing transvaginal prolapse repair with mesh. Obstet. Gynecol. Int. 2013:926313.
Fu, R. H., Wang, Y. C., Liu, S. P., Shih, T. R., Lin, H. L., Chen, Y. M., et al. (2014). Decellularization and recellularization technologies in tissue engineering. Cell Transplant. 23, 621–630. doi: 10.3727/096368914x678382
Galipeau, J., and Sensebe, L. (2018). Mesenchymal stromal cells: clinical challenges and therapeutic opportunities. Cell Stem Cell 22, 824–833. doi: 10.1016/j.stem.2018.05.004
Galipeau, J., Weiss, D. J., and Dominici, M. (2019). Response to Nature commentary “Clear up this stem-cell mess”. Cytotherapy 21, 1–2. doi: 10.1016/j.jcyt.2018.11.007
Gargett, C. E., Chan, R. W., and Schwab, K. E. (2008). Hormone and growth factor signaling in endometrial renewal: role of stem/progenitor cells. Mol. Cell. Endocrinol. 288, 22–29. doi: 10.1016/j.mce.2008.02.026
Gargett, C. E., and Gurung, S. (2016). Endometrial Mesenchymal stem/stromal cells, their fibroblast progeny in endometriosis, and more. Biol. Reprod. 94:129.
Gargett, C. E., Gurung, S., Darzi, S., Werkmeister, J. A., and Mukherjee, S. (2019). Tissue engineering approaches for treating pelvic organ prolapse using a novel source of stem/stromal cells and new materials. Curr. Opin. Urol 29, 450–457. doi: 10.1097/mou.0000000000000634
Gargett, C. E., Nguyen, H. P., and Ye, L. (2012). Endometrial regeneration and endometrial stem/progenitor cells. Rev. Endocr. Metab. Disord. 13, 235–251. doi: 10.1007/s11154-012-9221-9
Gargett, C. E., and Rogers, P. A. (2001). Human endometrial angiogenesis. Reproduction 121, 181–186. doi: 10.1530/rep.0.1210181
Gargett, C. E., Schwab, K. E., and Deane, J. A. (2016). Endometrial stem/progenitor cells: the first 10 years. Hum. Reprod. Update 22, 137–163.
Gargett, C. E., Schwab, K. E., Zillwood, R. M., Nguyen, H. P., and Wu, D. (2009). Isolation and culture of epithelial progenitors and mesenchymal stem cells from human endometrium. Biol. Reprod. 80, 1136–1145. doi: 10.1095/biolreprod.108.075226
Gargett, C. E., and Ye, L. (2012). Endometrial reconstruction from stem cells. Fertil. Steril. 98, 11–20. doi: 10.1016/j.fertnstert.2012.05.004
Gellersen, B., and Brosens, J. J. (2014). Cyclic decidualization of the human endometrium in reproductive health and failure. Endocr. Rev. 35, 851–905. doi: 10.1210/er.2014-1045
Ghobadi, F., Rahmanifar, F., Mehrabani, D., Tamadon, A., Dianatpour, M., Zare, S., et al. (2018). Endometrial mesenchymal stem stromal cells in mature and immature sheep: An in vitro study. Int. J. Reprod. Biomed. 16, 83–92.
Gronthos, S., Franklin, D. M., Leddy, H. A., Robey, P. G., Storms, R. W., and Gimble, J. M. (2001). Surface protein characterization of human adipose tissue-derived stromal cells. J. Cell. Physiol. 189, 54–63.
Gurung, S., Deane, J. A., Darzi, S., Werkmeister, J. A., and Gargett, C. E. (2018a). In vivo survival of human endometrial mesenchymal stem cells transplanted under the kidney capsule of immunocompromised mice. Stem Cells Dev. 27, 35–43. doi: 10.1089/scd.2017.0177
Gurung, S., Williams, S., Deane, J. A., Werkmeister, J. A., and Gargett, C. E. (2018b). The transcriptome of human endometrial Mesenchymal stem cells under TGFβR inhibition reveals improved potential for cell-based therapies. Front. Cell Dev. Biol. 6:164. doi: 10.3389/fcell.2018.00164
Gurung, S., Werkmeister, J. A., and Gargett, C. E. (2015). Inhibition of Transforming Growth Factor-beta Receptor signaling promotes culture expansion of undifferentiated human Endometrial Mesenchymal Stem/stromal Cells. Sci. Rep. 5:15042.
Han, X., Meng, X., Yin, Z., Rogers, A., Zhong, J., Rillema, P., et al. (2009). Inhibition of intracranial glioma growth by endometrial regenerative cells. Cell Cycle 8, 606–610. doi: 10.4161/cc.8.4.7731
Haniffa, M. A., Collin, M. P., Buckley, C. D., and Dazzi, F. (2009). Mesenchymal stem cells: the fibroblasts’ new clothes? Haematologica 94, 258–263. doi: 10.3324/haematol.13699
Hanna, J., Goldman-Wohl, D., Hamani, Y., Avraham, I., Greenfield, C., Natanson-Yaron, S., et al. (2006). Decidual NK cells regulate key developmental processes at the human fetal-maternal interface. Nat. Med. 12, 1065–1074. doi: 10.1038/nm1452
Hawkes, P. W. (2015). Fetal bovine serum: geographic origin and regulatory relevance of viral contamination. Bioresour. Bioprocess 2:34.
Hellstrom, M., Bandstein, S., and Brannstrom, M. (2017). Uterine tissue engineering and the future of uterus transplantation. Ann. Biomed. Eng. 45, 1718–1730. doi: 10.1007/s10439-016-1776-2
Hellstrom, M., Moreno-Moya, J. M., Bandstein, S., Bom, E., Akouri, R. R., Miyazaki, K., et al. (2016). Bioengineered uterine tissue supports pregnancy in a rat model. Fertil. Steril. 106:487-496.e481.
Hida, N., Nishiyama, N., Miyoshi, S., Kira, S., Segawa, K., Uyama, T., et al. (2008). Novel cardiac precursor-like cells from human menstrual blood-derived mesenchymal cells. Stem cells 26, 1695–1704. doi: 10.1634/stemcells.2007-0826
Jabbour, H. N., Kelly, R. W., Fraser, H. M., and Critchley, H. O. (2006). Endocrine regulation of menstruation. Endocr. Rev. 27, 17–46. doi: 10.1210/er.2004-0021
Jiang, X. X., Zhang, Y., Liu, B., Zhang, S. X., Wu, Y., Yu, X. D., et al. (2005). Human mesenchymal stem cells inhibit differentiation and function of monocyte-derived dendritic cells. Blood 105, 4120–4126. doi: 10.1182/blood-2004-02-0586
Jiang, Z., Hu, X., Yu, H., Xu, Y., Wang, L., Chen, H., et al. (2013). Human endometrial stem cells confer enhanced myocardial salvage and regeneration by paracrine mechanisms. J. Cell Mol. Med. 17, 1247–1260. doi: 10.1111/jcmm.12100
Jin, H. J., Bae, Y. K., Kim, M., Kwon, S.-J., Jeon, H. B., Choi, S. J., et al. (2013). Comparative analysis of human mesenchymal stem cells from bone marrow, adipose tissue, and umbilical cord blood as sources of cell therapy. Int. J. Mol. Sci. 14, 17986–18001. doi: 10.3390/ijms140917986
Kalkunte, S. S., Mselle, T. F., Norris, W. E., Wira, C. R., Sentman, C. L., and Sharma, S. (2009). Vascular endothelial growth factor C facilitates immune tolerance and endovascular activity of human uterine NK cells at the maternal-fetal interface. J. Immunol. 182, 4085–4092. doi: 10.4049/jimmunol.0803769
Kazemnejad, S., Akhondi, M.-M., Soleimani, M., Zarnani, A. H., Khanmohammadi, M., Darzi, S., et al. (2012). Characterization and chondrogenic differentiation of menstrual blood-derived stem cells on a nanofibrous scaffold. Int. J. Artif. Organs 35, 55–66. doi: 10.5301/ijao.5000019
Khanjani, S., Khanmohammadi, M., Zarnani, A.-H., Akhondi, M.-M., Ahani, A., Ghaempanah, Z., et al. (2014). Comparative evaluation of differentiation potential of menstrual blood-versus bone marrow-derived stem cells into hepatocyte-like cells. PLoS One 9:e86075. doi: 10.1371/journal.pone.0086075
Khanjani, S., Khanmohammadi, M., Zarnani, A.-H., Talebi, S., Edalatkhah, H., Eghtesad, S., et al. (2015). Efficient generation of functional hepatocyte-like cells from menstrual blood-derived stem cells. J. Tissue Eng. Regen. Med. 9, E124–E134.
Khanmohammadi, M., Khanjani, S., Bakhtyari, M. S., Zarnani, A. H., Edalatkhah, H., Akhondi, M. M., et al. (2012). Proliferation and chondrogenic differentiation potential of menstrual blood-and bone marrow-derived stem cells in two-dimensional culture. Int. J. Hematol. 95, 484–493. doi: 10.1007/s12185-012-1067-0
Khanmohammadi, M., Khanjani, S., Edalatkhah, H., Zarnani, A. H., Heidari-Vala, H., Soleimani, M., et al. (2014). Modified protocol for improvement of differentiation potential of menstrual blood-derived stem cells into adipogenic lineage. Cell Prolif. 47, 615–623. doi: 10.1111/cpr.12133
Kim, J., and Hematti, P. (2009). Mesenchymal stem cell-educated macrophages: a novel type of alternatively activated macrophages. Exp. Hematol. 37, 1445–1453. doi: 10.1016/j.exphem.2009.09.004
Kozhukharova, I., Zemelko, V., Kovaleva, Z., Alekseenko, L., Lyublinskaya, O., and Nikolsky, N. (2018). Therapeutic doses of doxorubicin induce premature senescence of human mesenchymal stem cells derived from menstrual blood, bone marrow and adipose tissue. Int. J. Hematol. 107, 286–296. doi: 10.1007/s12185-017-2346-6
Kuramoto, G., Shimizu, T., Takagi, S., Ishitani, K., Matsui, H., and Okano, T. (2018). Endometrial regeneration using cell sheet transplantation techniques in rats facilitates successful fertilization and pregnancy. Fertil. Steril. 110:172-181.e174.
Kuznetsov, S. A., Mankani, M. H., Gronthos, S., Satomura, K., Bianco, P., and Robey, P. G. (2001). Circulating skeletal stem cells. J. Cell Biol. 153, 1133–1140.
Lara, E., Rivera, N., Cabezas, J., Navarrete, F., Saravia, F., Rodriguez-Alvarez, L., et al. (2018). Endometrial stem cells in farm animals: potential role in uterine physiology and pathology. Bioengineering 5:75. doi: 10.3390/bioengineering5030075
Le Blanc, K., and Mougiakakos, D. (2012). Multipotent mesenchymal stromal cells and the innate immune system. Nat. Rev. Immunol. 12, 383–396. doi: 10.1038/nri3209
Letouzey, V., Tan, K. S., Deane, J. A., Ulrich, D., Gurung, S., Ong, Y. R., et al. (2015). Isolation and characterisation of mesenchymal stem/stromal cells in the ovine endometrium. PLoS One 10:e0127531. doi: 10.1371/journal.pone.0127531
Li, H. Y., Chen, Y. J., Chen, S. J., Kao, C. L., Tseng, L. M., Lo, W. L., et al. (2010). Induction of insulin-producing cells derived from endometrial mesenchymal stem-like cells. J. Pharmacol. Exp. Ther. 335, 817–829.
Li, W., and Ding, S. (2010). Generation of novel rat and human pluripotent stem cells by reprogramming and chemical approaches. Methods Mol. Biol. 636, 293–300. doi: 10.1007/978-1-60761-691-7_18
Liu, T., Huang, Y., Zhang, J., Qin, W., Chi, H., Chen, J., et al. (2014). Transplantation of human menstrual blood stem cells to treat premature ovarian failure in mouse model. Stem Cells Dev. 23, 1548–1557. doi: 10.1089/scd.2013.0371
Liu, Y., Niu, R., Yang, F., Yan, Y., Liang, S., Sun, Y., et al. (2018). Biological characteristics of human menstrual blood-derived endometrial stem cells. J. Cell Mol. Med. 22, 1627–1639. doi: 10.1111/jcmm.13437
Lucas, E. S., Dyer, N. P., Fishwick, K., Ott, S., and Brosens, J. J. (2016). Success after failure: the role of endometrial stem cells in recurrent miscarriage. Reproduction 152, R159–R166.
Luz-Crawford, P., Torres, M. J., Noel, D., Fernandez, A., Toupet, K., Alcayaga-Miranda, F., et al. (2016). The immunosuppressive signature of menstrual blood mesenchymal stem cells entails opposite effects on experimental arthritis and graft versus host diseases. Stem Cells 34, 456–469. doi: 10.1002/stem.2244
Mahdipour, E., Salmasi, Z., and Sabeti, N. (2019). Potential of stem cell-derived exosomes to regenerate β islets through Pdx-1 dependent mechanism in a rat model of type 1 diabetes. J. Cell. Physiol. 234, 20310–20321. doi: 10.1002/jcp.28631
Mahfoudi, A., Fauconnet, S., Bride, J., Beck, L., Remy-Martin, J. P., Nicollier, M., et al. (1992). Serum-free culture of stromal and functionally polarized epithelial cells of guinea-pig endometrium: a potential model for the study of epithelial-stromal paracrine interactions. Biol. Cell 74, 255–265. doi: 10.1016/0248-4900(92)90036-z
Manshadi, M. D., Navid, S., Hoshino, Y., Daneshi, E., Noory, P., and Abbasi, M. (2019). The effects of human menstrual blood stem cells-derived granulosa cells on ovarian follicle formation in a rat model of premature ovarian failure. Microsc. Res. Tech. 82, 635–642. doi: 10.1002/jemt.23120
Marinaro, F., Gómez-Serrano, M., Jorge, I., Silla-Castro, J. C., Vázquez, J., Sánchez-Margallo, F. M., et al. (2019). Unraveling the molecular signature of extracellular vesicles from endometrial-derived mesenchymal stem cells: potential modulatory effects and therapeutic applications. Front. Bioeng. Biotechnol. 7:431. doi: 10.3389/fbioe.2019.00431
Masuda, H., Anwar, S. S., Buhring, H. J., Rao, J. R., and Gargett, C. E. (2012). A novel marker of human endometrial mesenchymal stem-like cells. Cell Transplant. 21, 2201–2214. doi: 10.3727/096368911x637362
Menard, C., Dulong, J., Roulois, D., Hebraud, B., Verdiere, L., Pangault, C., et al. (2020). Integrated transcriptomic, phenotypic, and functional study reveals tissue-specific immune properties of mesenchymal stromal cells. Stem Cells 38, 146–159. doi: 10.1002/stem.3077
Meng, X., Ichim, T. E., Zhong, J., Rogers, A., Yin, Z., Jackson, J., et al. (2007). Endometrial regenerative cells: a novel stem cell population. J. Transl. Med. 5:57.
Miernik, K., and Karasinski, J. (2012). Porcine uterus contains a population of mesenchymal stem cells. Reproduction 143, 203–209. doi: 10.1530/rep-11-0202
Mitchell, J. B., McIntosh, K., Zvonic, S., Garrett, S., Floyd, Z. E., Kloster, A., et al. (2006). Immunophenotype of human adipose-derived cells: temporal changes in stromal-associated and stem cell-associated markers. Stem Cells 24, 376–385. doi: 10.1634/stemcells.2005-0234
Miyazaki, K., and Maruyama, T. (2014). Partial regeneration and reconstruction of the rat uterus through recellularization of a decellularized uterine matrix. Biomaterials 35, 8791–8800. doi: 10.1016/j.biomaterials.2014.06.052
Moll, G., Geissler, S., Catar, R., Ignatowicz, L., Hoogduijn, M. J., Strunk, D., et al. (2016). Cryopreserved or fresh mesenchymal stromal cells: only a matter of taste or key to unleash the full clinical potential of MSC Therapy? Adv. Exp. Med. Biol. 951, 77–98. doi: 10.1007/978-3-319-45457-3_7
Mukherjee, S., Darzi, S., Paul, K., Cousins, F. L., Werkmeister, J. A., and Gargett C. E. (2020). Electrospun nanofiber meshes with endometrial MSCs modulate foreign body response by increased angiogenesis, matrix synthesis, and anti-Inflammatory gene expression in mice: Implication in pelvic floor. Front. Paharmacol. 11:353. doi: 10.3389/fphar.2020.00353
Mukherjee, S., Darzi, S., Paul, K., Werkmeister, J. A., and Gargett, C. E. (2019a). Mesenchymal stem cell-based bioengineered constructs: foreign body response, cross-talk with macrophages and impact of biomaterial design strategies for pelvic floor disorders. Interface Focus 9:20180089. doi: 10.1098/rsfs.2018.0089
Mukherjee, S., Darzi, S., Rosamilia, A., Kadam, V., Truong, Y., Werkmeister, J. A., et al. (2019b). Blended Nanostructured Degradable Mesh with Endometrial Mesenchymal stem cells promotes tissue integration and anti-inflammatory response in vivo for pelvic floor application. Biomacromolecules 20, 454–468. doi: 10.1021/acs.biomac.8b01661
Murakami, K., Lee, Y. H., Lucas, E. S., Chan, Y. W., Durairaj, R. P., Takeda, S., et al. (2014). Decidualization induces a secretome switch in perivascular niche cells of the human endometrium. Endocrinology 155, 4542–4553. doi: 10.1210/en.2014-1370
Murphy, M. P., Wang, H., Patel, A. N., Kambhampati, S., Angle, N., Chan, K., et al. (2008). Allogeneic endometrial regenerative cells: an “Off the shelf solution” for critical limb ischemia? J. Transl. Med. 6:45. doi: 10.1186/1479-5876-6-45
Murray, L. M. A., and Krasnodembskaya, A. D. (2019). Concise review: intercellular communication via organelle transfer in the biology and therapeutic applications of stem cells. Stem Cells 37, 14–25. doi: 10.1002/stem.2922
Musina, R. A., Belyavski, A. V., Tarusova, O. V., Solovyova, E. V., and Sukhikh, G. T. (2008). Endometrial mesenchymal stem cells isolated from the menstrual blood. Bull. Exp. Biol. Med. 145, 539–543. doi: 10.1007/s10517-008-0136-0
Naji, A., Rouas-Freiss, N., Durrbach, A., Carosella, E. D., Sensebe, L., and Deschaseaux, F. (2013). Concise review: combining human leukocyte antigen G and mesenchymal stem cells for immunosuppressant biotherapy. Stem Cells 31, 2296–2303. doi: 10.1002/stem.1494
Nasef, A., Mathieu, N., Chapel, A., Frick, J., Francois, S., Mazurier, C., et al. (2007). Immunosuppressive effects of mesenchymal stem cells: involvement of HLA-G. Transplantation 84, 231–237. doi: 10.1097/01.tp.0000267918.07906.08
Nguyen, H. P. T., Sprung, C. N., and Gargett, C. E. (2012). Differential expression of Wnt signaling molecules between pre-and postmenopausal endometrial epithelial cells suggests a population of putative epithelial stem/progenitor cells reside in the basalis layer. Endocrinology 153, 2870–2883. doi: 10.1210/en.2011-1839
Nikoo, S., Ebtekar, M., Jeddi-Tehrani, M., Shervin, A., Bozorgmehr, M., Kazemnejad, S., et al. (2012). Effect of menstrual blood-derived stromal stem cells on proliferative capacity of peripheral blood mononuclear cells in allogeneic mixed lymphocyte reaction. J. Obstet. Gynaecol. Res. 38, 804–809. doi: 10.1111/j.1447-0756.2011.01800.x
Olalekan, S. A., Burdette, J. E., Getsios, S., Woodruff, T. K., and Kim, J. J. (2017). Development of a novel human recellularized endometrium that responds to a 28-day hormone treatment. Biol. Reprod. 96, 971–981. doi: 10.1093/biolre/iox039
Ong, Y. R., Cousins, F. L., Yang, X., Mushafi, A. A. A. A., Breault, D. T., Gargett, C. E., et al. (2018). Bone marrow stem cells do not contribute to endometrial cell lineages in chimeric mouse models. Stem Cells 36, 91–102. doi: 10.1002/stem.2706
Ordener, C., Cypriani, B., Vuillermoz, C., and Adessi, G. L. (1993). Epidermal growth factor and insulin induce the proliferation of guinea pig endometrial stromal cells in serum-free culture, whereas estradiol and progesterone do not. Biol. Reprod. 49, 1032–1044. doi: 10.1095/biolreprod49.5.1032
Padykula, H. A., Coles, L. G., Okulicz, W. C., Rapaport, S. I., McCracken, J. A., King, N. W., et al. (1989). The basalis of the primate endometrium: a bifunctional germinal compartment. Biol. Reprod. 40, 681–690. doi: 10.1095/biolreprod40.3.681
Patel, A. N., Park, E., Kuzman, M., Benetti, F., Silva, F. J., and Allickson, J. G. (2008). Multipotent menstrual blood stromal stem cells: isolation, characterization, and differentiation. Cell Transplant. 17, 303–311. doi: 10.3727/096368908784153922
Paul, K., Darzi, S., McPhee, G., Del Borgo, M. P., Werkmeister, J. A., Gargett, C. E., et al. (2019). 3D bioprinted endometrial stem cells on melt electrospun poly epsilon-caprolactone mesh for pelvic floor application promote anti-inflammatory responses in mice. Acta Biomater. 97, 162–176. doi: 10.1016/j.actbio.2019.08.003
Peron, J. P., Jazedje, T., Brandao, W. N., Perin, P. M., Maluf, M., Evangelista, L. P., et al. (2012). Human endometrial-derived mesenchymal stem cells suppress inflammation in the central nervous system of EAE mice. Stem Cell Rev. Rep. 8, 940–952. doi: 10.1007/s12015-011-9338-3
Prockop, D. J., Prockop, S. E., and Bertoncello, I. (2014). Are clinical trials with mesenchymal stem/progenitor cells too far ahead of the science? Lessons from experimental hematology. Stem Cells 32, 3055–3061. doi: 10.1002/stem.1806
Queckborner, S., Syk Lundberg, E., Gemzell-Danielsson, K., and Davies, L. C. (2020). Endometrial stromal cells exhibit a distinct phenotypic and immunomodulatory profile. Stem Cell Res. Ther. 11:15.
Rahimi, M., Zarnani, A. H., Mobini, S., Khorasani, S., Darzi, M., and Kazemnejad, S. (2018). Comparative effectiveness of three-dimensional scaffold, differentiation media and co-culture with native cardiomyocytes to trigger in vitro cardiogenic differentiation of menstrual blood and bone marrow stem cells. Biologicals 54, 13–21. doi: 10.1016/j.biologicals.2018.05.003
Rahimi, M., Zarnani, A.-H., Mohseni-Kouchesfehani, H., Soltanghoraei, H., Akhondi, M.-M., and Kazemnejad, S. (2014). Comparative evaluation of cardiac markers in differentiated cells from menstrual blood and bone marrow-derived stem cells in vitro. Mol. Biotechnol. 56, 1151–1162. doi: 10.1007/s12033-014-9795-4
Rajabi, Z., Yazdekhasti, H., Mugahi, S. M. H. N., Abbasi, M., Kazemnejad, S., Shirazi, A., et al. (2018). Mouse preantral follicle growth in 3D co-culture system using human menstrual blood mesenchymal stem cell. Reprod. Biol. 18, 122–131. doi: 10.1016/j.repbio.2018.02.001
Rajaraman, G., White, J., Tan, K. S., Ulrich, D., Rosamilia, A., Werkmeister, J., et al. (2013). Optimization and scale-up culture of human endometrial multipotent mesenchymal stromal cells: potential for clinical application. Tissue Eng. Part C Methods 19, 80–92. doi: 10.1089/ten.tec.2011.0718
Ren, G., Zhao, X., Zhang, L., Zhang, J., L’Huillier, A., Ling, W., et al. (2010). Inflammatory cytokine-induced intercellular adhesion molecule-1 and vascular cell adhesion molecule-1 in mesenchymal stem cells are critical for immunosuppression. J. Immunol. 184, 2321–2328. doi: 10.4049/jimmunol.0902023
Ren, H., Sang, Y., Zhang, F., Liu, Z., Qi, N., and Chen, Y. (2016). Comparative analysis of human Mesenchymal Stem Cells from Umbilical Cord, Dental Pulp, and Menstrual blood as sources for cell therapy. Stem Cells Int. 2016, 3516574.
Ren, H., Zhang, Q., Wang, J., and Pan, R. (2018). Comparative effects of umbilical cord-and menstrual blood-derived MSCs in repairing acute lung injury. Stem Cells Int. 2018:787362.
Rink, B. E., Beyer, T., French, H. M., Watson, E., Aurich, C., and Donadeu, F. X. (2018). The Fate of Autologous Endometrial Mesenchymal Stromal Cells After Application in the Healthy Equine Uterus. Stem Cells Dev. 27, 1046–1052. doi: 10.1089/scd.2018.0056
Roson-Burgo, B., Sanchez-Guijo, F., Del Canizo, C., and De Las Rivas, J. (2016). Insights into the human mesenchymal stromal/stem cell identity through integrative transcriptomic profiling. BMC Genomics 17:944. doi: 10.1186/s12864-016-3230-0
Rozemuller, H., Prins, H. J., Naaijkens, B., Staal, J., Buhring, H. J., and Martens, A. C. (2010). Prospective isolation of mesenchymal stem cells from multiple mammalian species using cross-reacting anti-human monoclonal antibodies. Stem Cells Dev. 19, 1911–1921. doi: 10.1089/scd.2009.0510
Samsonraj, R. M., Rai, B., Sathiyanathan, P., Puan, K. J., Rotzschke, O., Hui, J. H., et al. (2015). Establishing criteria for human mesenchymal stem cell potency. Stem Cells 33, 1878–1891. doi: 10.1002/stem.1982
Santamaria, X., Massasa, E. E., Feng, Y., Wolff, E., and Taylor, H. S. (2011). Derivation of insulin producing cells from human endometrial stromal stem cells and use in the treatment of murine diabetes. Mol. Ther. 19, 2065–2071. doi: 10.1038/mt.2011.173
Sato, K., Ozaki, K., Oh, I., Meguro, A., Hatanaka, K., Nagai, T., et al. (2007). Nitric oxide plays a critical role in suppression of T-cell proliferation by mesenchymal stem cells. Blood 109, 228–234. doi: 10.1182/blood-2006-02-002246
Schwab, K. E., Chan, R. W., and Gargett, C. E. (2005). Putative stem cell activity of human endometrial epithelial and stromal cells during the menstrual cycle. Fertil. Steril. 84, (Suppl. 2), 1124–1130. doi: 10.1016/j.fertnstert.2005.02.056
Schwab, K. E., and Gargett, C. E. (2007). Co-expression of two perivascular cell markers isolates mesenchymal stem-like cells from human endometrium. Hum. Reprod. 22, 2903–2911. doi: 10.1093/humrep/dem265
Schwab, K. E., Hutchinson, P., and Gargett, C. E. (2008). Identification of surface markers for prospective isolation of human endometrial stromal colony-forming cells. Hum. Reprod. 23, 934–943. doi: 10.1093/humrep/den051
Selmani, Z., Naji, A., Zidi, I., Favier, B., Gaiffe, E., Obert, L., et al. (2008). Human leukocyte antigen-G5 secretion by human mesenchymal stem cells is required to suppress T lymphocyte and natural killer function and to induce CD4+CD25highFOXP3+ regulatory T cells. Stem Cells 26, 212–222. doi: 10.1634/stemcells.2007-0554
Shahdadfar, A., Fronsdal, K., Haug, T., Reinholt, F. P., and Brinchmann, J. E. (2005). In vitro expansion of human mesenchymal stem cells: choice of serum is a determinant of cell proliferation, differentiation, gene expression, and transcriptome stability. Stem Cells 23, 1357–1366. doi: 10.1634/stemcells.2005-0094
Shi, G., Wang, G., Lu, S., Li, X., Zhang, B., Xu, X., et al. (2019). PD-L1 is required for human endometrial regenerative cells-associated attenuation of experimental colitis in mice. Am. J. Transl. Res. 11:4696.
Shokri, M. R., Bozorgmehr, M., Ghanavatinejad, A., Falak, R., Aleahmad, M., Kazemnejad, S., et al. (2019). Human menstrual blood-derived stromal/stem cells modulate functional features of natural killer cells. Sci. Rep. 9:10007.
Sipp, D., Robey, P. G., and Turner, L. (2018). Clear up this stem-cell mess. Nature 561, 455–457. doi: 10.1038/d41586-018-06756-9
Sivasubramaniyan, K., Harichandan, A., Schumann, S., Sobiesiak, M., Lengerke, C., Maurer, A., et al. (2013). Prospective Isolation of Mesenchymal Stem cells from human bone marrow using novel antibodies directed against sushi domain containing 2. Stem Cells Dev. 22, 1944–1954. doi: 10.1089/scd.2012.0584
Sobiesiak, M., Sivasubramaniyan, K., Hermann, C., Tan, C., Orgel, M., Treml, S., et al. (2010). The mesenchymal stem cell antigen MSCA-1 is identical to tissue non-specific alkaline phosphatase. Stem Cells Dev. 19, 669–677. doi: 10.1089/scd.2009.0290
Sotiropoulou, P. A., Perez, S. A., Gritzapis, A. D., Baxevanis, C. N., and Papamichail, M. (2006). Interactions between human mesenchymal stem cells and natural killer cells. Stem Cells 24, 74–85. doi: 10.1634/stemcells.2004-0359
Spitzer, T. L., Rojas, A., Zelenko, Z., Aghajanova, L., Erikson, D. W., Barragan, F., et al. (2012). Perivascular human endometrial mesenchymal stem cells express pathways relevant to self-renewal, lineage specification, and functional phenotype. Biol. Reprod. 86:58.
Su, K., Edwards, S. L., Tan, K. S., White, J. F., Kandel, S., Ramshaw, J. A. M., et al. (2014). Induction of endometrial mesenchymal stem cells into tissue-forming cells suitable for fascial repair. Acta Biomater. 10, 5012–5020. doi: 10.1016/j.actbio.2014.08.031
Subbarao, R. B., Shivakumar, S. B., Choe, Y. H., Son, Y. B., Lee, H. J., Ullah, I., et al. (2019). CD105(+) Porcine Endometrial Stromal Mesenchymal stem cells possess differentiation potential toward Cardiomyocyte-like cells and insulin-producing beta cell-like cells in vitro. Reprod. Sci. 26, 669–682. doi: 10.1177/1933719118786461
Sugawara, K., Hamatani, T., Yamada, M., Ogawa, S., Kamijo, S., Kuji, N., et al. (2014). Derivation of human decidua-like cells from amnion and menstrual blood. Sci. Rep. 4, 1–9.
Tamadon, A., Mehrabani, D., Zarezadeh, Y., Rahmanifar, F., Dianatpour, M., and Zare, S. (2017). Caprine Endometrial Mesenchymal Stromal Stem Cell: Multilineage Potential, Characterization, and Growth Kinetics in Breeding and Anestrous Stages. Vet. Med. Int. 2017:5052801.
Tan, J., Li, P., Wang, Q., Li, Y., Li, X., Zhao, D., et al. (2016). Autologous menstrual blood-derived stromal cells transplantation for severe Asherman’s syndrome. Hum. Reprod. 31, 2723–2729. doi: 10.1093/humrep/dew235
Thomas, H., Cowin, A. J., and Mills, S. J. (2017). The importance of Pericytes in healing: wounds and other pathologies. Int. J. Mol. Sci. 18:1129. doi: 10.3390/ijms18061129
Tiemann, T. T., Padma, A. M., Sehic, E., Backdahl, H., Oltean, M., Song, M. J., et al. (2020). Towards uterus tissue engineering: a comparative study of sheep uterus decellularisation. Mol. Hum. Reprod. 26, 167–178. doi: 10.1093/molehr/gaaa009
To, C., Farnsworth, R. H., Vail, M. E., Chheang, C., Gargett, C. E., Murone, C., et al. (2014). Hypoxia-controlled EphA3 marks a human endometrium-derived multipotent mesenchymal stromal cell that supports vascular growth. PLoS One 9:e112106. doi: 10.1371/journal.pone.0112106
Ulrich, D., Edwards, S. L., Su, K., Tan, K. S., White, J. F., Ramshaw, J. A., et al. (2014a). Human endometrial mesenchymal stem cells modulate the tissue response and mechanical behavior of polyamide mesh implants for pelvic organ prolapse repair. Tissue Eng. Part A 20, 785–798.
Ulrich, D., Tan, K. S., Schwab, K., Cheong, A., Deane, J. A., Rosamilia, A., et al. (2014b). Mesenchymal stem/stromal cells in postmenopausal endometrium. Hum. Reprod. 29, 1895–1905.
Ulrich, D., Gargett, C. E., Edwards, S., White, J., Rajaraman, G., Tan, K. S., et al. (2012). Human endometrial mesenchymal stem cells and novel meshes as an autologous cell-based therapy for pelvic organ prolapse (POP). Tissue Eng. Part A 6:125.
Ulrich, D., Muralitharan, R., and Gargett, C. E. (2013). Toward the use of endometrial and menstrual blood mesenchymal stem cells for cell-based therapies. Expert Opin. Biol. Ther. 13, 1387–1400. doi: 10.1517/14712598.2013.826187
Uzieliene, I., Urbonaite, G., Tachtamisevaite, Z., Mobasheri, A., and Bernotiene, E. (2018). The Potential of Menstrual Blood-Derived Mesenchymal stem cells for cartilage repair and regeneration: novel aspects. Stem Cells Int. 2018:5748126.
Vogel, W., Grunebach, F., Messam, C. A., Kanz, L., Brugger, W., and Buhring, H. J. (2003). Heterogeneity among human bone marrow-derived mesenchymal stem cells and neural progenitor cells. Haematologica 88, 126–133.
Wang, H., Jin, P., Sabatino, M., Ren, J., Civini, S., Bogin, V., et al. (2012). Comparison of endometrial regenerative cells and bone marrow stromal cells. J. Transl. Med. 10:207. doi: 10.1186/1479-5876-10-207
Wang, Y., Chen, X., Cao, W., and Shi, Y. (2014). Plasticity of mesenchymal stem cells in immunomodulation: pathological and therapeutic implications. Nat. Immunol. 15, 1009–1016. doi: 10.1038/ni.3002
Wolff, E. F., Gao, X. B., Yao, K. V., Andrews, Z. B., Du, H., Elsworth, J. D., et al. (2011). Endometrial stem cell transplantation restores dopamine production in a Parkinson’s disease model. J. Cell Mol. Med. 15, 747–755. doi: 10.1111/j.1582-4934.2010.01068.x
Wolff, E. F., Mutlu, L., Massasa, E. E., Elsworth, J. D., Eugene Redmond, D. Jr., and Taylor, H. S. (2015). Endometrial stem cell transplantation in MPTP- exposed primates: an alternative cell source for treatment of Parkinson’s disease. J. Cell Mol. Med. 19, 249–256. doi: 10.1111/jcmm.12433
Wu, X., Luo, Y., Chen, J., Pan, R., Xiang, B., Du, X., et al. (2014). Transplantation of human menstrual blood progenitor cells improves hyperglycemia by promoting endogenous progenitor differentiation in type 1 diabetic mice. Stem Cells Dev. 23, 1245–1257. doi: 10.1089/scd.2013.0390
Xiang, B., Chen, L., Wang, X., Zhao, Y., Wang, Y., and Xiang, C. (2017). Transplantation of menstrual blood-derived mesenchymal stem cells promotes the repair of LPS-induced acute lung injury. Int. J. Mol. Sci. 18:689. doi: 10.3390/ijms18040689
Xu, X., Li, X., Gu, X., Zhang, B., Tian, W., Han, H., et al. (2017). Prolongation of Cardiac allograft survival by endometrial regenerative cells: focusing on B-cell responses. Stem Cells Transl. Med. 6, 778–787. doi: 10.5966/sctm.2016-0206
Xu, X., Wang, Y., Zhang, B., Lan, X., Lu, S., Sun, P., et al. (2018). Treatment of experimental colitis by endometrial regenerative cells through regulation of B lymphocytes in mice. Stem Cell Res. Ther. 9:146.
Xu, Y., Shi, Y., and Ding, S. (2008). A chemical approach to stem-cell biology and regenerative medicine. Nature 453, 338–344. doi: 10.1038/nature07042
Yan, Z., Guo, F., Yuan, Q., Shao, Y., Zhang, Y., Wang, H., et al. (2019). Endometrial mesenchymal stem cells isolated from menstrual blood repaired epirubicin-induced damage to human ovarian granulosa cells by inhibiting the expression of Gadd45b in cell cycle pathway. Stem Cell Res. Ther. 10:4.
Yang, H. M., Sung, J. H., Choi, Y. S., Lee, H. J., Roh, C. R., Kim, J., et al. (2012). Enhancement of the immunosuppressive effect of human adipose tissue-derived mesenchymal stromal cells through HLA-G1 expression. Cytotherapy 14, 70–79. doi: 10.3109/14653249.2011.613926
Yang, X., Devianti, M., Yang, Y. H., Ong, Y. R., Tan, K. S., Gurung, S., et al. (2019). Endometrial mesenchymal stem/stromal cell modulation of T cell proliferation. Reproduction 157, 43–52. doi: 10.1530/rep-18-0266
Yang, X. Y., Wang, W., and Li, X. (2014). In vitro hepatic differentiation of human endometrial stromal stem cells. In Vitro. Cell Dev. Biol. Anim. 50, 162–170. doi: 10.1007/s11626-013-9688-z
Yin, M., Zhou, H. J., Lin, C., Long, L., Yang, X., Zhang, H., et al. (2019). CD34+KLF4+ Stromal Stem Cells Contribute to Endometrial Regeneration and Repair. Cell Rep 27:2709-2724.e2703.
Young, N., Rosamilia, A., Arkwright, J., Lee, J., Davies-Tuck, M., Melendez, J., et al. (2017). Vaginal wall weakness in parous ewes: a potential preclinical model of pelvic organ prolapse. Int. Urogynecol. J. 28, 999–1004. doi: 10.1007/s00192-016-3206-2
Zafardoust, S., Kazemnejad, S., Darzi, M., Fathi-Kazerooni, M., Rastegari, H., and Mohammadzadeh, A. (2020). Improvement of pregnancy rate and live birth rate in poor ovarian responders by Intraovarian Administration of autologous menstrual blood derived- Mesenchymal Stromal Cells: Phase I/II Clinical Trial. Stem Cell Rev. Rep. doi: 10.1007/s12015-020-09969-6 [Epub ahead of print].
Zemel’ko, V. I., Grinchuk, T. M., Domnina, A. P., Artsybasheva, I. V., Zenin, V. V., Kirsanov, A. A., et al. (2011). Multipotent mesenchymal stem cells of desquamated endometrium: isolation, characterization and use as feeder layer for maintenance of human embryonic stem cell lines. Tsitologiia 53, 919–929.
Zhang, S., Zeng, N., Alowayed, N., Singh, Y., Cheng, A., Lang, F., et al. (2017). Downregulation of endometrial mesenchymal marker SUSD2 causes cell senescence and cell death in endometrial carcinoma cells. PLoS One 12:e0183681. doi: 10.1371/journal.pone.0183681
Zhang, Y., Lin, X., Dai, Y., Hu, X., Zhu, H., Jiang, Y., et al. (2016). Endometrial stem cells repair injured endometrium and induce angiogenesis via AKT and ERK pathways. Reproduction 152, 389–402. doi: 10.1530/rep-16-0286
Zhao, Y., Chen, X., Wu, Y., Wang, Y., Li, Y., and Xiang, C. (2018a). Transplantation of human menstrual blood-derived mesenchymal stem cells alleviates Alzheimer’s disease-like pathology in APP/PS1 transgenic mice. Front. Mol. Neurosci. 11:140. doi: 10.3389/fnmol.2018.00140
Zhao, Y., Lan, X., Wang, Y., Xu, X., Lu, S., Li, X., et al. (2018b). Human endometrial regenerative cells attenuate bleomycin-induced pulmonary fibrosis in mice. Stem Cells Int. 2018, 3475137.
Keywords: endometrium, menstrual blood, culture expansion, perivascular MSC, eMSC, MenSC, cell therapy, immunomodulation
Citation: Bozorgmehr M, Gurung S, Darzi S, Nikoo S, Kazemnejad S, Zarnani A-H and Gargett CE (2020) Endometrial and Menstrual Blood Mesenchymal Stem/Stromal Cells: Biological Properties and Clinical Application. Front. Cell Dev. Biol. 8:497. doi: 10.3389/fcell.2020.00497
Received: 31 March 2020; Accepted: 25 May 2020;
Published: 09 July 2020.
Edited by:
Lindolfo da Silva Meirelles, Universidade Luterana do Brazil, BrazilReviewed by:
Francesco De Francesco, Azienda Ospedaliero Universitaria Ospedali Riuniti, ItalyRachel Chan, The University of Hong Kong, Hong Kong
Copyright © 2020 Bozorgmehr, Gurung, Darzi, Nikoo, Kazemnejad, Zarnani and Gargett. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Amir-Hassan Zarnani, zarnania@tums.ac.ir; Caroline E. Gargett, caroline.gargett@hudson.org.au
†These authors have contributed equally to this work
 Mahmood Bozorgmehr
Mahmood Bozorgmehr Shanti Gurung
Shanti Gurung Saeedeh Darzi
Saeedeh Darzi Shohreh Nikoo6†
Shohreh Nikoo6†  Amir-Hassan Zarnani
Amir-Hassan Zarnani Caroline E. Gargett
Caroline E. Gargett