Abstract
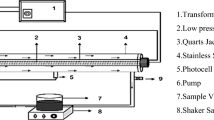
This study provides new insight into the implementation and synergy effect of coupling in sequences of the photocatalysis ([TiO2-UVC]), ozone (O3) and hydrogen peroxide (H2O2) applied to degradation and mineralization of a model compound such as caffeine (in synthetic solution and industrial wastewater). The system consists of an annular photoreactor coupled to two venturi valves with a continuous dosage of O3 and H2O2, the combination of binary processes such as O3/H2O2, TiO2/O3 and UVC/H2O2 was evaluated, as well as the coupling of TiO2/O3/H2O2/UVC in order to estimate all the possible synergy effects. It was found that the coupling between photocatalysis and hydrogen peroxide shows a negligible performance of caffeine treatment in both synthetic and real wastewater because the reaction rate, degradation and mineralization of caffeine does not improve considerably compared to the photocatalysis process alone. Meanwhile, the coupling between photocatalysis and ozonation shows the highest synergy effect. The coupling of all the processes evaluated TiO2/O3/H2O2/UVC achieved 100% degradation of caffeine, but the mineralization was lower than photocatalysis coupled with ozonation. This could be due to the peroxide and ozone compete by active sites of the catalyst and can also act as scavengers of oxidizing species, hindering the caffeine mineralization. The results show that the combination of photocatalysis with ozone and peroxide can make a synergy effect for industrial wastewater treatment.











Similar content being viewed by others
References
Peña-Guzmán C, Ulloa-Sánchez S, Mora K et al (2019) Emerging pollutants in the urban water cycle in Latin America: a review of the current literature. J Environ Manage 237:408–423. https://doi.org/10.1016/j.jenvman.2019.02.100
Chávez AM, Quiñones DH, Rey A et al (2020) Simulated solar photocatalytic ozonation of contaminants of emerging concern and effluent organic matter in secondary effluents by a reusable magnetic catalyst. Chem Eng J. https://doi.org/10.1016/j.cej.2020.125642
Ling Y, Liao G, Xie Y et al (2016) Coupling photocatalysis with ozonation for enhanced degradation of Atenolol by Ag-TiO2 micro-tube. J Photochem Photobiol A 329:280–286. https://doi.org/10.1016/j.jphotochem.2016.07.007
Wang Y, Liu H, Liu G et al (2015) Kinetics for diclofenac degradation by chlorine dioxide in aqueous media: Influences of natural organic matter additives. J Taiwan Inst Chem Eng 56:131–137. https://doi.org/10.1016/j.jtice.2015.04.015
Evaluation A (2018) Photocatalytic degradation of commercial acetaminophen: evaluation, modeling, and scaling-up of photoreactors. Catalysts. https://doi.org/10.3390/catal8050179
Poulios I, Micropoulou E, Panou R, Kostopoulou E (2003) Photooxidation of eosin Y in the presence of semiconducting oxides. Appl Catal B 41:345–355. https://doi.org/10.1016/S0926-3373(02)00160-1
Lachheb H, Puzenat E, Houas A et al (2002) Photocatalytic degradation of various types of dyes (Alizarin S, Crocein Orange G, Methyl Red, Congo Red, Methylene Blue) in water by UV-irradiated titania. Appl Catal B 39:75–90. https://doi.org/10.1016/S0926-3373(02)00078-4
Toepfer B, Gora A, Lipuma G (2006) Photocatalytic oxidation of multicomponent solutions of herbicides: reaction kinetics analysis with explicit photon absorption effects. Appl Catal B 68:171–180. https://doi.org/10.1016/j.apcatb.2006.06.020
Kuo WS, Chiang YH, Lai LS (2006) Degradation of carbofuran in water by solar photocatalysis in presence of photosensitizers. J Environ Sci Health Part B 41:937–948. https://doi.org/10.1080/03601230600806137
Valério A, Wang J, Tong S et al (2020) Synergetic effect of photocatalysis and ozonation for enhanced tetracycline degradation using highly macroporous photocatalytic supports. Chem Eng Process-Process Intensif 149:107838. https://doi.org/10.1016/j.cep.2020.107838
Pham TD, Lee BK (2015) Disinfection of Staphylococcus aureus in indoor aerosols using Cu-TiO2 deposited on glass fiber under visible light irradiation. J Photochem Photobiol A 307–308:16–22. https://doi.org/10.1016/j.jphotochem.2015.04.002
Pham TD, Lee BK (2017) Novel photocatalytic activity of Cu@V co-doped TiO2/PU for CO2 reduction with H2O vapor to produce solar fuels under visible light. J Catal 345:87–95. https://doi.org/10.1016/j.jcat.2016.10.030
Pham TD, Lee BK (2016) Advanced removal of C. famata in bioaerosols by simultaneous adsorption and photocatalytic oxidation of Cu-doped TiO2/PU under visible irradiation. Chem Eng J 286:377–386. https://doi.org/10.1016/j.cej.2015.10.100
Diaz-Angulo J, Porras J, Mueses M et al (2019) Coupling of heterogeneous photocatalysis and photosensitized oxidation for diclofenac degradation: role of the oxidant species. J Photochem Photobiol A 383:112015. https://doi.org/10.1016/j.jphotochem.2019.112015
Pham TD, Lee BK (2015) Novel integrated approach of adsorption and photo-oxidation using Ag-TiO2/PU for bioaerosol removal under visible light. Chem Eng J 275:357–365. https://doi.org/10.1016/j.cej.2015.04.055
Lara-Ramos JA, Sánchez-Gómez K, Valencia-Rincón D et al (2019) Intensification of the O3/TiO2/UV advanced oxidation process using a modified flotation cell. Photochem Photobiol Sci 18:920–928. https://doi.org/10.1039/c8pp00308d
Dionysiou DD, Suidan MT, Baudin I, Laîné JM (2004) Effect of hydrogen peroxide on the destruction of organic contaminants-synergism and inhibition in a continuous-mode photocatalytic reactor. Appl Catal B 50:259–269. https://doi.org/10.1016/j.apcatb.2004.01.022
Otálvaro-Marín HL, González-Caicedo F, Arce-Sarria A et al (2019) Scaling-up a heterogeneous H2O2/TiO2/solar-radiation system using the DamkÖhler number. Chem Eng J 364:244–256. https://doi.org/10.1016/j.cej.2019.01.141
Teodosiu C, Gilca AF, Barjoveanu G, Fiore S (2018) Emerging pollutants removal through advanced drinking water treatment: a review on processes and environmental performances assessment. J Clean Prod 197:1210–1221. https://doi.org/10.1016/j.jclepro.2018.06.247
Ameta R, Ameta SC (2017) Photocatalysis: principles and applications, 1st edn. CRC Press, London
Rivas FJ, Beltrán FJ, Encinas A (2012) Removal of emergent contaminants: Integration of ozone and photocatalysis. J Environ Manage 100:10–15. https://doi.org/10.1016/j.jenvman.2012.01.025
Kim SJ, Kim SC, Seo SG et al (2011) Photocatalyzed destruction of organic dyes using microwave/UV/O3/H2O2/TiO2 oxidation system. Catal Today 164:384–390. https://doi.org/10.1016/j.cattod.2010.10.025
Fernandes A, Gągol M, Makoś P et al (2019) Integrated photocatalytic advanced oxidation system (TiO2/UV/O3/H2O2)for degradation of volatile organic compounds. Sep Purif Technol 224:1–14. https://doi.org/10.1016/j.seppur.2019.05.012
Sahel K, Elsellami L, Mirali I et al (2016) Hydrogen peroxide and photocatalysis. Appl Catal B 188:106–112. https://doi.org/10.1016/j.apcatb.2015.12.044
Hossain MK, Mortuza AA, Sen SK et al (2018) A comparative study on the influence of pure anatase and Degussa-P25 TiO2 nanomaterials on the structural and optical properties of dye sensitized solar cell (DSSC) photoanode. Optik (Stuttg) 171:507–516. https://doi.org/10.1016/j.ijleo.2018.05.032
Diaz J, Gomez-Bonilla I, Jimenez-Tohapanta C et al (2019) Visible-light activation of TiO2 by dye-sensitization for degradation of pharmaceutical compounds. Photochem Photobiol Sci 18:897–904. https://doi.org/10.1039/c8pp00270c
Koltsakidou AM, Evgenidou E et al (2017) Cytarabine degradation by simulated solar assisted photocatalysis using TiO2. Chem Eng J 316:823–831. https://doi.org/10.1016/j.cej.2017.01.132
Wang KH, Hsieh YH, Wu CH, Chang CY (2000) The pH and anion effects on the heterogeneous photocatalytic degradation of o-methylbenzoic acid in TiO2 aqueous suspension. Chemosphere 40:389–394. https://doi.org/10.1016/S0045-6535(99)00252-0
Colina-Márquez J, Machuca-Martínez F, Puma GL (2010) Radiation absorption and optimization of solar photocatalytic reactors for environmental applications. Environ Sci Technol 44:5112–5120. https://doi.org/10.1021/es100130h
Otálvaro-Marín HL, Mueses MA, Machuca-Martínez F (2014) Boundary layer of photon absorption applied to heterogeneous photocatalytic solar flat plate reactor design. Int J Photoenergy. https://doi.org/10.1155/2014/930439
Qian R, Zong H, Schneider J et al (2019) Charge carrier trapping, recombination and transfer during TiO2 photocatalysis: an overview. Catal Today 335:78–90. https://doi.org/10.1016/j.cattod.2018.10.053
da Costa Filho BM, Araujo ALP, Padrão SP et al (2019) Effect of catalyst coated surface, illumination mechanism and light source in heterogeneous TiO2 photocatalysis using a mili-photoreactor for n-decane oxidation at gas phase. Chem Eng J 366:560–568. https://doi.org/10.1016/j.cej.2019.02.122
Marien CBD, Le Pivert M, Azaïs A et al (2019) Kinetics and mechanism of Paraquat’s degradation: UV-C photolysis vs UV-C photocatalysis with TiO2/SiC foams. J Hazard Mater 370:164–171. https://doi.org/10.1016/j.jhazmat.2018.06.009
Diaz-Angulo J, Lara-Ramos J, Mueses M et al (2020) Enhancement of the oxidative removal of diclofenac and of the TiO2 rate of photon absorption in dye-sensitized solar pilot scale CPC photocatalytic reactors. Chem Eng J 381:122520. https://doi.org/10.1016/j.cej.2019.122520
Elhalil A, Elmoubarki R, Farnane M et al (2018) Photocatalytic degradation of caffeine as a model pharmaceutical pollutant on Mg doped ZnO-Al2O3 heterostructure. Environ Nanotechnol Monit Manag 10:63–72. https://doi.org/10.1016/j.enmm.2018.02.002
Awfa D, Ateia M, Fujii M et al (2018) Photodegradation of pharmaceuticals and personal care products in water treatment using carbonaceous-TiO2 composites: A critical review of recent literature. Water Res 142:26–45. https://doi.org/10.1016/j.watres.2018.05.036
Zhao L, Ma J, Sun Z, Liu H (2009) Mechanism of heterogeneous catalytic ozonation of nitrobenzene in aqueous solution with modified ceramic honeycomb. Appl Catal B 89:326–334. https://doi.org/10.1016/j.apcatb.2008.12.009
Addamo M, Augugliaro V, García-López E et al (2005) Oxidation of oxalate ion in aqueous suspensions of TiO2 by photocatalysis and ozonation. Catal Today 107–108:612–618. https://doi.org/10.1016/j.cattod.2005.07.030
Domínguez JR, Beltrán J, Rodríguez O (2005) Vis and UV photocatalytic detoxification methods (using TiO2, TiO2/H2O2, TiO2/O3, TiO2/S2O82−, O3, H2O2, S2O82−. Catal Today 101:389–395. https://doi.org/10.1016/j.cattod.2005.03.010
Alaton I, Balcioglu IA, Bahnemann D (2002) Advanced oxidation of a reactive dyebath effluent:comparison of O3, H2O2/UV-C and TiO2/UV-A processes. Water Res 36:1143–1154
Rueda-Marquez JJ, Levchuk I, Fernández Ibañez P, Sillanpää M (2020) A critical review on application of photocatalysis for toxicity reduction of real wastewaters. J Clean Prod 258: https://doi.org/10.1016/j.jclepro.2020.120694
Beltrán FJ (2003) Ozone reaction kinetics for water and wastewater systems, 1st edn. London, CRC Press
Valdés H, Zaror CA (2006) Heterogeneous and homogeneous catalytic ozonation of benzothiazole promoted by activated carbon: kinetic approach. Chemosphere 65:1131–1136. https://doi.org/10.1016/j.chemosphere.2006.04.027
Malik SN, Ghosh PC, Vaidya AN, Mudliar SN (2020) Hybrid ozonation process for industrial wastewater treatment: principles and applications. A review. J Water Process Eng. https://doi.org/10.1016/j.jwpe.2020.101193
Cortez Vásquez A, Rosales Gerónimo G, Naupari Quiroz R, Vega Huerta H (2016) Photocatalysis fundamentals and perspectives, Laurence M. RSC Energy and Environment Series
Hirakawa T, Nosaka Y (2002) Properties of O2.- and OH. Formed in TiO2 aqueous suspensions by photocatalytic reaction and the influence of H2O2 and some ions. Langmuir 18:3247–3254. https://doi.org/10.1021/la015685a
Quiñones DH, Álvarez PM, Rey A et al (2015) Application of solar photocatalytic ozonation for the degradation of emerging contaminants in water in a pilot plant. Chem Eng J 260:399–410. https://doi.org/10.1016/j.cej.2014.08.067
Conway DC (1957) Mechanism of the homogeneous decomposition of hydrogen peroxide. J Phys Chem 61:1579–1580. https://doi.org/10.1021/j150557a034
Monteagudo JM, Durán A, San Martín I (2014) Mineralization of wastewater from the pharmaceutical industry containing chloride ions by UV photolysis of H2O2/Fe(II) and ultrasonic irradiation. J Environ Manage 141:61–69. https://doi.org/10.1016/j.jenvman.2014.03.020
Acknowledgements
The authors thank the COLCIENCIAS for funding the national doctoral program (727-2015 and 647-2014); Universidad del Valle and Tecnoparque-SENA Nodo-Cali (Grant No. I2020-031-1093).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lara-Ramos, J.A., Llanos-Diaz, G.D., Diaz-Angulo, J. et al. Evaluation of Caffeine Degradation by Sequential Coupling of TiO2/O3/H2O2/UV Processes. Top Catal 63, 1361–1373 (2020). https://doi.org/10.1007/s11244-020-01316-w
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11244-020-01316-w