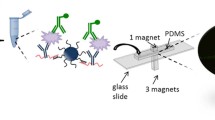
Abstract
The enhanced capture of the antigen (Ag) by the surface-immobilized antibodies (Ab) in heterogeneous microfluidic immunosensors lowers (improves) the detection limit thus facilitating the early disease detection. The efficient capture of the antigen is subjected to the interaction of the transport parameters, reaction parameters and the microfluidic system geometry. In the present work, the role of active mixing in facilitating the transport in flow-based heterogeneous immunosensor was studied, both numerically and experimentally. The experiments were performed with the capture of prostate-specific antigen (PSA) by anti-PSA and the results were validated using numerical simulations. The average Sherwood number (Sh) for the experiments and simulations was comparable. First, the effect of the nature of the flow on the capture efficiency was tested, which showed the enhancement in the antigen capture using active mixing. Further, studies of individual active mixing parameter (average velocity, frequency and amplitude) on analyte capture were conducted. The increase in average velocity (uavg) increases the antigen capture but the corresponding pressure drop also increases, and the figure of merit (FOM) attains a maximum at Re ~ 0.6. Similar behavior was observed with the increase of the frequency. The continuous increase in the frequency reduces the reaction time scale and decreases the analyte capture. The amplitude has the least effect on the antigen capture. The flow profiles were also seen and the temporal variation was compared at different flow conditions with micro-PIV. We also proposed two correlations utilizing the parameters—the average surface concentration (Cs,avg), the Reynolds number (Re) and the Strouhal number (St). The correlations indicate that the dependency coefficient for St is higher than Re, and signify the impact of vortex propagation (i.e., active mixing) on antigen capture.









Similar content being viewed by others
References
Abbott MSR, Harvey AP, Perez GV et al (2012) Biological processing in oscillatory baffled reactors: operation, advantages and potential. Interface Focus 3:1–13
Abbott MSR, Harvey AP, Perez GV et al (2014) Reduced power consumption compared to a traditional stirred tank reactor (STR) for enzymatic saccharification of alpha-cellulose using oscillatory baffled reactor (OBR) technology. Chem Eng Res Des 92:1969–1975
Alonso MB, Granados X, Faraudo J et al (2014) Magnetic actuator for the control and mixing of magnetic bead-based reactions on-chip. Anal Bioanal Chem 406:6607–6616
Art MS, Noblitt SD, Krummel AT et al (2018) IR-compatible PDMS microfluidic devices for monitoring of enzyme kinetics. Anal Chim Acta 1021:95–102
Asgharzadehahmadi S, Raman AAA, Parthasarathy R et al (2016) Sonochemical reactors: review on features, advantages and limitations. Renew Sustain Energy Rev 63:302–314
Bange A, Halsall B, Heineman WR (2005) Microfluidic immunosensor systems. Biosens Bioelectr 20:2488–2503
Bhuvana M, Narayanan JS, Dharuman V et al (2013) Gold surface supported spherical liposome–gold nano-particle nano-composite for label free DNA sensing. Biosens Bioelectr 41:802–808
Brown AP, Anson FC (1977) Cyclic and differential pulse voltammetric behavior of reactants confined to the electrode surface. Anal Chem 49:1589–1595
Chauhan R, Singh J, Solanki PR (2016) Label-free piezoelectric immunosensor decorated with gold nanoparticles: kinetic analysis and biosensing application. Sens Actutors B 222:804–814
Chen CK, Cho CC (2008) A combined active/passive scheme for enhancing the mixing efficiency of microfluidic devices. Chem Eng Sci 63:3081–3087
Chen Q, Wang D, Cai G et al (2016) Fast and sensitive detection of foodborne pathogen using electrochemical impedance analysis, urease catalysis and microfluidics. Biosens Bioelectr 86:770–776
Chen S et al (2019) Microfluidic device directly fabricated on screen-printed electrodes for ultrasensitive electrochemical sensing of PSA. Nano Res Lett 14:71
Chepyala R, Panda S (2013) Tunable surface free energies of functionalized molecular layers on Si surfaces for microfluidic immunosensor applications. Appl Surf Sci 271:77–85
Chepyala R, Panda S (2014) Zeta potential and reynolds number correlations for electrolytic solutions in microfluidic immunosensor. Micro Nano 18:1329–1339
Cherkasov N, Denissenko P, Deshmukh S et al (2020) Gas-liquid hydrogenation in continuous flow- The effect of mass transfer and residence time in powder packed-bed and catalyst-coated reactors. Chem Eng J 379:122292
Di CM, Ting YY, Yang DZ et al (2019) Microchannel with stacked microbeads for separation of plasma from whole blood. Chin J Anal Chem 47(5):661–668
From MathWorld– A Wolfram Web Resource. http://mathworld.wolfram.com/PiecewiseFunction.html. Accessed 22 Feb 2020
Fujii T (2002) PDMS-based microfluidic devices for biomedical applications. Micro Elec Eng 61–62:907–914
Fujii T, Sando Y, Higashino K et al (2003) A plug and play microfluidic device. Lab Chip 3:193–197
Glasgow I, Lieber S, Aubry N (2004) Parameters influencing pulsed flow mixing in microchannels. Anal Chem 76:4825–4832
Hart R, Lec R, Noh HM (2010) Enhancement of heterogeneous immunoassays using AC electroosmosis. Sens Actatuars B 147:366–375
Hessel V, Lowe H, Schonfeld F (2005) Micromixers—a review on passive and active mixing principles. Chem Eng Sci 60:2479–2501
Hu G, Gao Y, Li D (2007) Modeling micropatterned antigen-antibody binding kinetics in a microfluidic chip. Biosens Bioelectr 22:1403–1409
Ibii T, Kaieda M, Hatakeyama S et al (2010) Direct immobilization of gold-binding antibody fragments for immunosensor applications. Anal Chem 82:4229–4235
Imran H, Manikandan PN, Prabhu D et al (2019) Ultra selective label free electrochemical detection of cancer prognostic p53-antibody at DNA functionalized grapheme. Sens Biosens Res 23:100261
Kadilak AL, Ying L, Shrestha S et al (2014) Selective deposition of chemically- bonded gold electrodes onto PDMS microchannel side walls. J Electr Anal Chem 727:141–147
Kalita P, Singh J, Singh MK et al (2012) Ring like self-assembled Ni nanoparticles based biosensor for food toxin detection. Appl Phys Lett 100:093702
Kardous F, Fissi LE, Friedt JM et al (2011) Integrated active mixing and biosensing using low frequency vibrating mixer and Love-wave sensor for real time detection of antibody binding event. J Appl Phys 109(094701):1–8
Klink MJ, Iwuoha EI, Ebenso EE (2011) The electro-catalytic and redox-mediator effects of nanostructured PDMA-PSA modified-electrodes as phenol derivative sensors. Int J Electrochem Sci 6:2429–2442
Kumar H, Tawhai MH, Hoffmann EA et al (2011) Steady streaming: a key mixing mechanism in low-Reynolds-number acinar flows. Phys Fluids 23:041902-1–04190222
Lebedev K, Mafe S, Stroeve P (2006) Convection, diffusion and reaction in a surface-based biosensor: modeling of cooperativity and binding site competition on the surface and in the hydrogel. J Colloid Interface Sci 296:527–537
Lee CY, Chang CL, Wang YN et al (2011) Microfluidic mixing: a review. Int J Mol Sci 12:3263–3287
Mandal MM, Kumar V, Nigam KDP (2010) Augmentation of heat transfer performance in coiled flow inverter vis-a-vis conventional heat exchanger. Chem Eng Sci 65:999–1007
Mandal MM, Palka A, Nigam KDP (2011) Liquid_Liquid mixing in coiled flow inverter. Ind Eng Chem Res 50:13230–13235
Mansur EA, Mingxing YE, Yundong W et al (2008) A State-of-the-art review of mixing in microfluidic mixers. Chin J Chem Engg 16(4):503–516
Masngut N, Harvey AP (2012) Intensification of biobutanol production in batch oscillatory baffled bioreactor. Proc Eng 42:1079–1087
Nelson KE, Foley JO, Yager P (2007) Concentration Gradient Immunoassay. 1. An immunoassay based on interdiffusion and surface binding in a microchannel. Anal Chem 79:3542–3548
Nguyen NT, Wu Z (2005) Micromixers—a review. J Micromech Microeng 15:R1–R16
Ni X, Gao S, Pritchard DW (1994) A study of mass transfer in yeast in a pulsed baffled. Bioreactor Biotech Bioeng 45:165–175
Ni X, Gao S, Pritchard DW et al (1995) A comparative study of mass transfer in yeast for a batch pulsed baffled bioreactor and a stirred tank fermenter. Chem Eng Sci 50(13):2127–2136
Rath D, Panda S (2015) Enhanced capture efficiencies of antigens in immunosensors. Chem Eng J 60:657–670
Rath D, Panda S (2016) Correlation of capture efficiency with the geometry, transport, and reaction parameters in heterogeneous immunosensors. Langmuir 32:1410–1418
Rath D, Kumar S, Panda S (2012) Enhancement of antigen–antibody kinetics on nanotextured silicon surfaces in mixed non-flow systems. Mater Sci Eng C 32:2223–2229
Renaudin A, Chabot V, Grondin E et al (2010) Integrated active mixing and biosensing using surface acoustic waves (SAW) and surface plasmon resonance (SPR) on a common substrate. Lab Chip 10:111–115
Reverberi R, Reverberi L (2007) Factors affecting the antigen-antibody reaction. Blood Transfus 5:227–240
Sarvazyan A (2010) Diversity of biomedical applications of acoustic radiation force. Ultrasonics 50:230–234
Singh J, Kalita P, Singh MK et al (2011) Nanostructured nickel oxide-chitosan film for application to cholesterol sensor. Appl Phys Fluid 98:123702
Singh J, Choudhary N, Nigam KDP (2014) The thermal and transport characteristics of nanofluids in a novel three-dimensional device. Can J Chem Eng 92:2185–2201
Solanki PR, Kaushik A, Ansari AA et al (2008) Zinc oxide-chitosan nanobiocomposite for urea sensor. Appl Phys Lett 93:163903
Squires TM, Messinger RJ, Manalis SR (2008) Making it stick: convection, reaction and diffusion in surface-based biosensors. Nat Biol 26:417–426
Srivastava S et al (2011) A self assembled monolayer based microfluidic sensor for ures detection. Nanoscale 3:2971–2977
Subramanian RS Convective mass transfer. Department of Chemical and Biomolecular Engineering, Clarkson University. https://web2.clarkson.edu/projects/subramanian/ch330/notes/Convective%20Mass%20Transfer.pdf Accessed on 3 June 2020
Verma S, Panda S (2020) Correlations of the capture efficiency with the Dean number and its constituents in heterogeneous microfluidic immunosensors. Micro Nano 24:9
Vijayendran RA, Leckband DE (2001) A quantitative assessment of heterogeneity for surface-immobilized proteins. Anal Chem 73:471–480
Wang AW, Kiwan R, White RM et al (1998) A silicon-based ultrasonic immunoassay for detection of breast cancer antigens. Sens Actaturs B 49:13–21
Wu G, Datar RH, Hansen KM et al (2001) Bioassay of prostate-specific antigen (PSA) using microcantilevers. Biotech Nat 19:856–860
Yu C, Kim GB, Clark PM et al (2015) A microfabricated quantum dot-linked immuno-diagnostic assay (µQLIDA) with an electrohydrodynamic mixing element. Sens Actaturs B 209:722–728
Zhang F et al (2015) A Microfluidic love-wave biosensing device for PSA detection based on an aptamer. Beacon Probe Sensors 15:13839–13850
Zhang D et al (2019) Electrochemical aptamer-based microsensor for real-time monitoring of adenosine in vivo. Anal Chimica Acta 1076:55–63
Acknowledgements
Financial support from the Ministry of Electronics and Information Technology, Government of India (Grant Number 2(4)/2014-PEGD (IPIIW)) is acknowledged. The use of the micro-PIV unit (supported by the FIST program of the Department of Science and Technology, Government of India) in the PG Research Laboratory in the Department of Chemical Engineering is acknowledged. Also, the help of Dr. Satyendra Kumar with the processing of the antibody and antigen samples is acknowledged.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Verma, S., Panda, S. Effect of active mixing on capture efficiency in heterogeneous microfluidic immunosensor. Microfluid Nanofluid 24, 58 (2020). https://doi.org/10.1007/s10404-020-02364-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10404-020-02364-0