Abstract
Cherry tomatoes are one of the most popular tomato varieties known for their bioactive compounds and sensory properties. One way to reduce the contamination of tomato is to coat them with natural or antimicrobial substances. In this study, an ethanolic extract of propolis (EEP) was obtained, and its chemical composition was analyzed using high-performance liquid chromatography with diode array detector (HPLC-DAD), and minimum inhibitory concentration (MIC) and the minimum bactericidal/fungicidal concentration (MBC/MFC) were determined using the serial microdilution method. The antimicrobial activity of 5 and 10% EEP and pullulan films containing EEP (5 and 10%) against Listeria monocytogenes, Salmonella Typhimurium, Escherichia coli O157, Penicillium chrysogenum, Fusarium solani, and Botrytis cinerea were compared. The influence of a pullulan coating containing EEP (5 and 10%) on reducing the number of bacteria and molds, physicochemical properties (weight loss (WL), total soluble solids (TSS), titratable acidity (TA), maturity index, pH, and color), and sensory properties (color and brightness of skin, aroma, flavor, overall quality, and general preference) of cherry tomatoes during refrigerated storage was evaluated. Pullulan films with EEP inhibited the growth of microorganisms on cherry tomatoes. These coatings did not affect the TSS and pH values of tomatoes, but a slight decrease in TA and WL was observed. Cherry tomatoes coated with pullulan coatings containing EEP did not show any adverse changes in their sensory properties. It was found that the addition of EEP to the pullulan coatings enriched them with antimicrobial properties and enhanced their action while reducing the WL and maturation time of cherry tomatoes.

Graphical Abstract
Similar content being viewed by others
Introduction
Tomatoes are one of the most popular vegetables in the world. These annual, self-pollinating plants belong to the Solanaceae family. Shortly after the discovery of America, tomatoes were brought to Europe and grown all over the world (Terzopoulos and Bebeli 2010). Today, they are eaten fresh as well as in processed forms such as ketchup, pastes, and sauces. Tomatoes contain many bioactive compounds, and their chemical composition includes vitamins C and E, carotenoids, chlorophyll, lycopene, organic acids, flavonoids, and phenols, which have pro-health effects such as reducing the incidence of heart disease (Asensio et al. 2019; Mahieddine et al. 2018). One of the most popular varieties of tomatoes is cherry tomatoes, which are aromatic, red-colored, and round-shaped with a hard texture and small size (Madureira et al. 2019), and known worldwide for their nutritional value and taste (Lin et al. 2019).
Tomatoes are easily contaminated by microbes which use them as raw material, and together with peppers, these have been identified as a potential carrier of foodborne pathogens. Between 1998 and 2016, as many as 8857 diseases were reported associated with the consumption of these vegetables in the USA. These were identified to be caused by Salmonella sp., Shigella sp., Listeria monocytogenes, Campylobacter jejuni, Staphylococcus aureus, Clostridium perfringens, and Bacillus cereus (Cabrera-Díaz et al. 2018). A study by Zhou et al. confirmed that microbiological contamination of tomatoes can occur at any stage of production and processing. The most common cause of contamination is the impure irrigation water, contaminated pesticide solutions, or contaminated machinery and water used for fruit washing during the stage of packaging (Zhou et al. 2018). Bacterial pathogens, including Salmonella, are easily absorbed through the wounds of tomatoes, scars on stems, and flowers which provide them more opportunities to survive or grow, while at the same time making it harder to deactivate them without affecting the sensory characteristics of vegetables (Yuk et al. 2006). Tomatoes are also very susceptible to fungal pathogens, and those that commonly infect this vegetable are Botrytis cinerea (which causes gray mold), Fusarium solani (which causes root rot), and Alternaria solani (which causes brown spots on the fruit) (Tang et al. 2019).
There is a search for effective methods for the elimination of pathogens from tomatoes. The most commonly used agent for washing and disinfecting tomatoes is sodium hypochlorite (NaOCl). However, it does not facilitate the complete removal of pathogenic and spoilage microorganisms (Chang and Schneider 2012). The possibility of using for grape tomatoes of washing solution with thymol, sodium dodecyl sulfate, acetic acid, and H2O2 to reduce Salmonella enterica Typhimurium was found (Lu et al. 2014). Preservatives approved for food, such as acidified sodium benzoate, are used for the microbiological protection of tomatoes (Chen and Zhong 2018). A study reported successful inactivation of Escherichia coli O157:H7 and S. enterica on grape tomatoes by applying an integrated treatment of ultraviolet (UV) light and low-dose gamma irradiation (Mukhopadhyay et al. 2013). It has also been proven that the use of pulsed light effectively inactivated E. coli and Listeria innocua on tomato slices (Valdivia-Nájar et al. 2017). An effective but economically inefficient method to inactivate foodborne pathogens is to use superheated steam at temperatures above 150 °C for a few seconds, which was shown to result in a reduction of E. coli O157:H7, S. Typhimurium, and L. monocytogenes by more than 5 log CFU g−1 on cherry tomatoes (Ban and Kang 2018). In addition to preservatives, certain natural substances are also researched for protecting tomatoes against microbial growth. Some of these include sumac water extracts, oregano oil, and essential oil of Adansonia digitata (Gündüz et al. 2010; Kayode et al. 2018). One of the natural substances that prevents the growth of B. cinerea is trans-cinnamaldehyde, which inhibits the growth of gray mold on cherry tomatoes by up to 60% (Guo et al. 2019).
Propolis is a mixture of resinous substances collected by honey bees from leaves, stems, and flower buds. The chemical composition of propolis differs depending on the harvest region or season. Propolis contains over 400 active substances, such as aromatic acids, esters, amino acids, microelements, and vitamins. In addition, propolis has phenolic compounds that play an important role in its antimicrobial properties. These are aromatic, secondary metabolites of plants, and provide protection against pathogens and UV radiation (Pobiega et al. 2017; Ripari et al. 2019). Research has shown that propolis has many biological properties including antimicrobial, antifungal, and antiviral functions and inhibits the oxidation of nutrients in food (Asawahame et al. 2015). Due to the presence of bioactive ingredients in abundance, propolis has a great potential to extend the shelf life of various food products. It can be used as a preservative and to prevent adverse physicochemical changes (Pobiega et al. 2019b). However, as it contains a high amount of wax and other physical impurities, including bee and frame fragments, it is necessary to use propolis as an alcoholic or aqueous extract in technological applications. Moreover, due to its intense aroma and flavor, propolis extracts may cause sensory changes in food. Therefore, the effects of using propolis extracts on the surface of food or as an additive in edible films that are used as food coatings have been more carefully studied (Jansen-Alves et al. 2019; Pobiega et al. 2019b).
Pullulan is a water-soluble exopolysaccharide of microbiological origin produced by the fungus Aureobasidium pullulans. It is a linear α-D-glucan, composed of maltotriose subunits connected by 1-6-α-D-glycosidic bonds (Gniewosz and Duszkiewicz-Reinhard 2008). The molecular weight of pullulan varies from 4.5 × 104 to 6 × 105 Da (Cheng et al. 2011). Due to its physical and chemical properties, pullulan is widely researched for industrial applications. It has been used in various industries such as food, medical, pharmaceutical, and cosmetic (Ma et al. 2014). Pullulan can be used for forming edible coatings and films, which are colorless, odorless, nontoxic, and biodegradable, and therefore works well as a food packaging material (Niu et al. 2019). After drying, pullulan films form thin, brittle membranes with no flavor or aroma. Due to their brittleness, it is necessary to modify these films by adding an appropriate plasticizer that can increase their resistance to stretching and bending. Glycerol, fatty acid esters, and proteins are most commonly used for this purpose (Kraśniewska et al. 2019). However, pullulan films can adhere well to the surface of a product and exhibit high mechanical strength. They are also resistant to fats and act as a barrier to oxygen (Rekha and Sharma 2007). Moreover, pullulan coatings can be easily removed from food when washed under running water (Kraśniewska et al. 2019).
The combination of propolis and pullulan may act as a novel natural agent for protecting food against pathogens and microorganisms causing food spoilage. Therefore, in this study, the properties of pullulan films supplemented with propolis extracts were analyzed. Due to the promising results of earlier work in this study, we undertook to check the antimicrobial action of a pullulan coating containing ethanol propolis extract to extend the postharvest life of cherry tomato. To our knowledge, this is the first study on this subject.
Materials and Methods
Propolis Extraction Method
Propolis was purchased from an apiary located in Toruń County (53.03′ N, 18.62′ E) in Poland. Raw samples of propolis were frozen (− 20 °C) and mechanically ground. Samples (100 g) of pulverized crude propolis were extracted with a 10-fold volume of 70% ethanol solution and were subsequently subjected to ultrasound. Then, the samples were treated with an Omni Ruptor 4000 sonicator equipped with a titanium microtip (Omni Ruptor 4000, OMNI International−The Homogenizer Company, Kennesaw, GA, USA). The sonication process was carried out for 20 min at a power of 210 W and a frequency of 20 kHz in ice and water baths, and the samples were shaken (200 rpm) at 28 °C for 1 day (SM−30 Control, Edmund Bühler, Germany). The obtained dry extracts were filtered by gravity filtration on a Whatman No. 4 filter (Millipore, USA) and condensed under reduced pressure at 40 °C (Rotavapor R 215, Büchi, Switzerland). The ethanol extract of propolis (EEP) was concentrated to dryness by evaporating the solvent, and then, the working solutions (density 280 g L−1) were prepared in 70% ethanol. The prepared EEP samples were stored at 4 °C (Pobiega et al. 2019a, c).
High-Performance Liquid Chromatography with Diode Array Detector (HPLC-DAD) Analysis Parameters
Method Validation
Commercially available standards (Merck, Darmstadt, Germany) and ChromaDex® (Irvine, USA) were separately dissolved with methanol (Sigma-Aldrich, Poznań, Poland) in a 25-mL volumetric flask according to the ChromaDex’s Tech Tip 0003: reference standard recovery and dilution and used as standard stock solutions. Working solutions for calibration were prepared by diluting 10 and 100 μL of standard stock solutions with methanol in 10-mL volumetric flasks, 500 and 1000 μL in 5-mL volumetric flasks, and 1000 μL in 2-mL volumetric flasks. The working solutions and undiluted stock solutions were injected (1 μL) on a column in six replicates (n = 6) using an autosampler SIL-20AC HT to generate a six-point calibration curve according to the external standard method by correlating concentration with signal peak area. Method parameters are calculated with Microsoft (Microsoft Corporation, Redmond, USA) (Table 1). Signal-to-noise ratio approach was used to determine the limit of detection (LOD) (S/N of 3:1) and limit of quantification (LOQ) (S/N of 10:1).
Parameters of Separations
All extracts were filtered with Iso-Disc™ Filters PTFE-25-2, diameter 25 mm, pore size 0.20 μm (Supelco Analytical™, Bellefonte, USA). A Shimadzu Prominence chromatograph equipped with an autosampler SIL-20AC HT, photodiode array detector SPD-M20A, and LC solution 1.21 SP1 chromatography software (Shimadzu, Kyoto, Japan) were used. Separations were performed at C18 reversed phase, 2.6-μm particles with solid core and porous outer layer, 100 × 4.60 mm column (Kinetex™, Phenomenex®, Torrance, USA). Binary gradient of deionized water (WCA R03 DP ECO, COBRABiD Aqua, Warsaw, Poland) adjusted to pH 2 with phosphoric acid (Merck) and filtered with 0.20 μm, 47-mm diameter nylon membrane filter (Phenex™, Phenomenex®, Torrance, USA) and MeCN (acetonitrile for HPLC ≥ 99.9%, Merck) was used as follows: 0 min, 12.5% B; 25.0 min, 40% B; 34.0 min, 60% B; 37.0 min, 95% B; 37.1 min, 12.5% B; 40 min, stop; flow rate 2.0 mL/min; oven temperature 45 °C; and injection volume 1 μL. Peak identification was carried out by comparison of retention time as well UV spectra with standards. The content of the determined compounds was calculated in mg L−1 of EEP (Pobiega et al. 2019c).
Strains and Inocula Preparation
The cultures of Listeria monocytogenes ATCC 7644, Salmonella enterica serovar Typhimurium (NIPH-NIH), Escherichia coli O157 ATCC 700728, Penicillium chrysogenum ATCC 10136, Fusarium solani ATCC 36031, and Botrytis cinerea IOR 2110 were provided by the Department of Food Biotechnology and Microbiology (WULS-SGGW, Poland).
The bacterial strains were cultured on Müller Hinton agar (MHA) and incubated at 37 °C ± 1 °C for 24 h. Bacterial inocula were prepared in sterile 0.85% NaCl (w/v) solution to achieve a population of approximately 1 × 107 CFU mL−1. The mold spores were obtained from mycelia grown on Sabouraud agar (SA) after incubation at 22 °C for 14 days. Fungal suspensions were prepared in sterile 0.85% NaCl to achieve 1 × 106 spores mL−1. The number of spores was calculated using a hemocytometer.
Determination of Minimum Inhibitory Concentration and Minimum Bactericidal/Fungicidal Concentration
The minimum inhibitory concentration (MIC) and minimum bactericidal/fungicidal concentration (MBC/MFC) of EEP were determined using the serial microdilution method (Balouiri et al. 2016; Machado et al. 2016; Zargaran et al. 2017). Dilutions of EEP were prepared in Mueller-Hinton Broth (MHB) and RPMI 1640 medium at a concentration range of 320–5 g L−1. Then, 20 μL of EEP from each dilution and 10 μL of bacterial/fungal suspensions were separately transferred to 96-well plates (Profilab, Poland). In each well, 170 μL of MHB (for bacteria) or RPMI 1640 (for fungi) was added. The final volume of each well was 200 μL, and the final EEP concentration was in the range of 32–0.5 g L−1. The final concentration of the bacterial inoculum was 5 × 105 CFU mL−1 and that of the mold was 5 × 104 CFU mL−1. The plates with bacteria were incubated at 37 °C for 24 h and those with molds at 28 °C for 72 h. After incubation, 15 μL of aqueous resazurin solution (resazurin sodium salt C12H6NNaO4 with a final concentration of 0.675 g L−1), which was metabolically reduced by active cells to a colored derivative, was added to the wells to allow the visual identification of metabolic activity. The development of a purple-pink color was considered as indicative of bacterial or fungal growth. MIC was estimated as the lowest concentration of the EEP at which the purple-pink color was not observed and expressed in mg mL−1. The analysis was repeated 3 times.
To determine the MBC and MFC of propolis extract, 100 μL of bacterial/fungal culture from each well, in which no growth was observed, was reinoculated onto MHA (for bacteria) or SA (for fungi) plates and was incubated at 37 °C for 24 h (for bacteria) or at 28 °C for 72 h (for fungi). After incubation, the plates were checked for the growth of colonies. MBC/MFC was determined in vitro as the concentration of EEP at which 99.9% of bacterial or fungal cells were killed, or the number of bacteria or fungi was reduced by 3 logarithmic cycles. The values were expressed in mg mL−1.
Disc Diffusion Method
The sensitivity of the tested microorganisms to EEP was studied using the disc diffusion method (Standards Institute Clinical Laboratory (CLSI) 2006). Briefly, sterile cellulose discs of 6 mm diameter were impregnated with 9 or 18 μL of EEP to obtain a constant amount of the dry matter of EEP (2.5 and 5 mg disc−1, respectively). Bacterial (1 × 108 CFU mL−1) and fungal suspensions (1 × 106 CFU mL−1) were applied to the MHA surface. Next, three EEP-impregnated discs were placed on the inoculated media. Bacterial plates were incubated at 37 °C for 24 h and fungal plates at 28 °C for 72 h. Slide calipers were used to measure the diameter of the zones of inhibition of the strain growth without subtracting the disc diameter. The result was expressed in mm.
Preparation of Pullulan Films with EEP
Two EEP pullulan films were prepared with the following chemical composition (g L−1): pullulan (Hayashibara Co., Ltd., Japan) 100, glycerol 10, Tween-80 10, and EEP 50 (P + 5% EEP) or 100 (P + 10% EEP). Control pullulan film was prepared with the following chemical composition (g L−1): pullulan 100, glycerol 10, and Tween-80 10 (P). After mixing the solution at 80 °C for 15 min, it was cooled, and then, 10 mL was transferred to Petri dishes with 90 mm diameter and dried at 35 °C for 18 h. The films were then stored at 25 °C and 50% relative humidity (RH).
Determination of the Antimicrobial Activity of Films
Antimicrobial properties of pullulan films prepared with EEP were examined using the disc diffusion method. Briefly, discs of 6 mm diameter were cut out of the prepared films using a circular knife. Bacterial suspensions (1 × 108 CFU mL−1) were applied to the MHA surface and fungal suspensions (1 × 106 CFU mL−1) to SA. Next, three discs were placed on the inoculated media. The pullulan film disc prepared without EEP was used as a negative control. Bacterial plates were incubated at 37 °C for 24 h and fungal plates at 28 °C for 72 h. Slide calipers were used to measure the diameter of the zones of inhibition of the strain growth without subtracting the disc diameter. The result was expressed in mm.
Preparation of Coating for Tomatoes
The whole fresh, undamaged cherry tomatoes (Solanum lycopersicum var. cerasiforme) weighing 10 g ± 0.2 g were purchased from a local market and stored at 4 °C and 80% RH. They were washed with a 0.05% sodium hypochlorite solution for 3 min to reduce the natural microflora. After disinfection, the tomatoes were rinsed in sterile distilled water and dried for about 1 h in a laminar chamber. Coating solution was prepared with the following chemical composition (g L−1): pullulan 10, glycerol 10, Tween-80 10, and EEP 50 or 100. Coating solution was applied to tomatoes with an airbrush cake decorating device (PZ-270XS with a 0.5-mm nozzle, PointZero Airbrush Co., USA) in 2 layers, and then, the tomatoes were dried at room temperature for 2 h in a laminar chamber. In this way, 4 groups of tomatoes were prepared with 20 each: control tomatoes (C), pullulan-coated tomatoes (P), tomatoes coated with pullulan containing 5% EEP (P + 5% EEP), and tomatoes coated with pullulan containing 10% EEP (P + 10% EEP). The tomatoes were stored at 10 °C for 21 days in a laboratory thermostat with RH ranging from 58 to 63% and then used for physicochemical analyses. The experiments were performed in 3 independent replicates.
Evaluation of Coating Effectiveness Against Bacteria and Molds
Washed and dried cherry tomatoes were inoculated with bacterial and mold strains. On the scar sites of tomatoes, 10 μL of inoculum containing about 1 × 108 CFU mL−1 of bacteria or about 1 × 106 CFU mL−1 of mold spores was applied using a pipette. The tomatoes were placed in Petri dishes and left in a biosafety cabinet (Esco, Singapore) at room temperature for 3 h to improve the adhesion of bacterial cells or mold spores to the stem scars. Then, they were treated with coating solutions prepared as described above. Inoculated tomatoes were stored at 10 °C for 21 days.
During storage, the number of bacteria and molds on tomatoes was tested. Tomatoes weighing 10 g ± 0.2 g were transferred to bags with 90 mL of saline and homogenized (Stomacher 400 Circulator, UK) for 30 s. A series of tenfold dilutions in saline solution was made from the homogenized material. Then, 0.1 mL of dilutions (in duplicate) was cultured on selective media: Palcam agar (PA) for L. monocytogenes, Hektoen agar (HA) for E. coli, xylose lysine deoxycholate (XLD) agar for S. Typhimurium, and dichloran rose bengal chloramphenicol agar (DRBCA) for molds. PA, HA, and XLD agar plates were incubated at 37 °C for 48 h and DRBCA plates at 28 °C for 72 h. The number of microorganisms was determined in CFU g−1 at 0, 7, 14, and 21 days in 3 repetitions.
Determination of Weight Losses of Tomatoes
The weight of tomatoes was determined using an analytical scale (AS 220X, Radwag, Poland) on days 0, 7, 14, and 21. Weight loss was expressed as a percentage in relation to the initial weight.
Determination of Total Soluble Solids (TSS), Titratable Acidity (TA), Maturity Index (MI), and pH of Tomatoes
Samples of tomatoes were blended into a smooth mass, and the pH of the obtained solution was measured. TSS was determined by refractometry (electric refractometer, Atago, Japan), and the value of soluble solids was expressed in percent. TA was determined in the tomato mass diluted with water (1:10) by titrating with 0.1 M NaOH to pH 8.1 using an automatic titrator (Mettler Toledo, Warsaw, Poland). TA was calculated as the content of malic acid. Both TSS and TA were determined at 0, 7, 14, and 21 days of storage in three repetitions. MI was determined as the ratio of TSS to TA.
Determination of Tomatoes Color
Color was measured with spectrophotometer CM-5 (Konica-Minolta, UK), equipped with a D65 light source, using the L*a*b* reflective method with a standard observer setting as 2°. Cherry tomatoes were placed on a 5-mm measuring gap for color determination, and the measurement was performed in ten repetitions. The total color change (ΔE) was calculated from the following formula:
where ∆L∗, ∆a∗, and ∆b∗ represent the difference in brightness L*, coordinate a*, and coordinate b* between coated tomato tissue and control tomato tissue at the same time, respectively.
Sensory Evaluation
Tomatoes were prepared as in item 2.8. Sensory evaluation of coated and uncoated tomatoes stored in the laboratory thermostat at 10 °C was carried out after 48 h. Samples after removing from the thermostat were left at room temperature for about 2 h. The flavor of the tomatoes was tested after washing them under running water. The sensory properties were evaluated by a team of 50 untrained panelists (tomato eaters), who were students and staff at the Faculty of Food Technology (WULS-SGGW), aged 21–56 years. The panelists assessed the following characteristics of tomatoes: color and brightness of skin, natural and foreign aroma, natural and foreign flavor, overall quality, and general preference of tomatoes. A 5-point scale was used for evaluation, where 1 means the lowest and 5 the highest grade.
Statistical Analysis
Data were presented as mean ± SD. Three replications were carried out for each experiment. Statistical tests were performed by using the Statistica version 13 PL (TIBCO, Palo Alto, USA). Multivariate correlation analysis was used for the evaluation of the spectrum–effect relationships. One-way analysis of variance was carried out. The significance of differences between mean values was assessed using the Tukey test or the Kruskal–Wallis test at a significance level of p < 0.05.
Results and Discussion
Chemical Composition of EEP
HPLC analysis shows the presence of 22 chemical components in EEP, belonging mainly to the groups of flavonoids and polyphenols (Table 1 and Fig. 1). The total content of the identified flavonoids was 63,130 mg L−1 and that of phenolic acids was 20,030 mg L−1. The group of flavonoids included compounds belonging to the following classes: flavanones, flavanoles, flavones, flavonoles, and flavanes. The highest content among flavonoids was flavones (26,267.2 mg L−1) and flavanones (21,704.7 mg L−1). Among flavonoids, chrysin, pinocembrin, galangin, pinobanksin, and pinostrobin were determined as present in the highest amount, while among phenolic acids, caffeic, p-coumaric, and ferulic acids were the highest.
HPLC-DAD chromatogram of ethanolic extract of propolis. (1) 3,4-Dihydroxybenzoic acid; (2) (+)-catechin; (3) 4-hydroxybenzoic acid; (4) caffeic acid; (5) vanillic acid; (6) syringic acid; (7) p-coumaric acid; (8) ferulic acid; (9) cichoric acid; (10) dimethyl caffeic acid; (11) cinnamyl alcohol; (12) cinnamic acid; (13) 4-methoxycinnamic acid; (14) quercetin; (15) pinobanksin; (16) apigenin; (17) kaempferol; (18) isorhamnetin; (19) chrysin; (20) pinocembrin; (21) galangin; (22) (±)-pinostrobin
The chemical composition of propolis depends on the climate and geographical location from where it is obtained (Bankova 2005). So far, more than 400 chemical components have been identified in various types of propolis (de Groot et al. 2014). Each type of propolis has marker components that allow it to be classified as a given type. The propolis we studied belongs to the poplar type, and its characteristic chemical components include pinocembrin, chrysin, pinostrobin, and p-coumaric acid (Isidorov et al. 2014; Kuś et al. 2018; Popova et al. 2017). Other types of propolis are dominated by different chemical components; for example, Brazilian red propolis contains luteolin, naringenin, kaempferol, pinocembrin, and biochanin A; Pacific propolis contains prenylflavanones, namely, propolin H, propolin G, propolin D, propolin C, and propolin F; and Mediterranean propolis contains isocupressic, pimaric, and imbricatoloic acid (Graikou et al. 2016; Machado et al. 2016; Popova et al. 2010). The applied method for the preparation of extracts of propolis allows to obtain EEP with a high content of bioactive compounds (Pobiega et al. 2019a). Earlier studies of Polish propolis extracts showed similar chemical composition and content of individual components (Pobiega et al. 2019c; Grecka et al. 2019).The antimicrobial activity of propolis is caused by the synergistic effects of all its chemical components, especially flavonoids and phenolic compounds that dominate in the chemical composition (Wolska et al. 2016). Different classes of flavonoids exhibit different mechanisms of action on microbial cells; for example, flavonol classes of compounds destroy the cytoplasmic membrane, while flavonol, flavan-3-ol, and flavone classes of compounds inhibit the process of energy metabolism (Ahmad et al. 2015). Therefore, regardless of the type and origin, propolis is believed to possess antimicrobial effects.
Minimum Inhibitory Concentration and Minimum Bactericidal/Fungicidal Concentration of EEP
Antimicrobial and antifungal activities of EEP were studied using classical techniques by determining the values of MIC, MBC, and MFC. The corresponding results are presented in Table 2. The MIC value of propolis extract was determined at concentrations ranging from 2 mg mL−1 for L. monocytogenes, P. chrysogenum, F. solani, and B. cinerea to 8 mg mL−1 for S. Typhimurium and E. coli. The MBC value was higher for S. Typhimurium and E. coli (16 mg mL−1) than for the other tested strains (8 mg mL−1).
EEP is the most frequently tested solvent for different industrial applications. A very wide range of MIC values was found for different EEPs with respect to Salmonella (32–14,700 μg mL−1) and E. coli (16–5000 μg mL−1), while EEP from Turkey and Oman were identified to show higher antimicrobial activity (Przybyłek and Karpiński 2019). Grecka et al. (2019) determined that the MIC of Polish EEP was greater than 4096 μg mL−1 for E. coli. In a study on Taiwanese propolis, the MIC of extracts was determined to be 40 μg mL−1 against L. monocytogenes and greater than 640 μg mL−1 against E. coli (Y. W. Chen et al. 2018). An Egyptian study on EEP determined an MIC value equal to 1 mg mL−1 for E. coli, 1.1 mg mL−1 for S. enterica, and 0.2 mg mL−1 for L. monocytogenes (Mohdaly et al. 2015). Our previous studies indicated a stronger effect of EEP on Gram-positive bacteria than Gram-negative bacteria, which is confirmed by the results observed in the present study (Pobiega et al. 2019c). Few studies are available on the antimold effect of EEP. Yang et al. (2016) showed that the Chinese EEP completely inhibited the growth of B. cinerea mold at a concentration of 0.8 mg mL−1, and Galletti et al. (2017) showed a significant inhibitory effect of EEP on F. solani biofilms.
Comparison of Antimicrobial Activity of EEP and Pullulan Films with EEP
The antimicrobial activity of EEP was compared with that of pullulan films with EEP using the disc diffusion method. The control pullulan film did not show any antimicrobial activity. EEP and pullulan films with EEP had larger zones of inhibition of test strain growth than the disc diameter only against L. monocytogenes and among molds against F. solani and P. chrysogenum (Table 3). In the case of S. Typhimurium and E. coli, no growth was observed under the discs, and for B. cinerea, no inhibition of growth was observed. P + 5% EEP film caused statistically significantly (p < 0.05) larger inhibitory zones for L. monocytogenes than for other microorganisms. On the other hand, P + 10% EEP film inhibited L. monocytogenes and F. solani more strongly compared with the other tested strains.
Moreover, it is found that EEP and P + 5% EEP and P + 10% EEP films inhibited the growth of L. monocytogenes bacteria and F. solani mold with equal power, which was indicated by the lack of statistically significant differences (p > 0.05) between the size of the zones (Table 3). In the case of P. chrysogenum, a weaker effect of pullulan films with EEP than the extract itself was observed. This phenomenon may indicate the limited diffusion of some active components of propolis from the pullulan film matrix to the substrate. In general, the diffusion of active components of propolis (i.e., polyphenols and flavonoids) depends on the film matrix. Mascheroni et al. (2010) found that polyphenols are released in larger amounts from polylactic acid film than flavonoids, which remained in the polymer. By contrast, beads of chitosan-containing propolis were able to release the flavonoids from propolis (Mascheroni et al. 2014).
According to previous research, a pullulan film does not have antimicrobial properties in itself, and only due to the addition of natural substances such as the extract of meadowsweet flowers or sweet basil, or essential oil from oregano, caraway, or rosemary, it gains antimicrobial and antifungal properties (Gniewosz et al. 2013; Kraśniewska et al. 2016; Morsy et al. 2014; Synowiec et al. 2014a, b). Similarly, the cassava starch film was found to gain antimicrobial activity after the addition of EEP (De Araújo et al. 2015), while the incorporation of 10% EPP to chitosan coating onto the food contact surface of synthetic polymer films enhanced their antibacterial activity against L. monocytogenes, E. coli O157:H7, S. aureus, Cronobacter sakazakii, B. cereus, and S. Typhimurium (Torlak and Sert 2013).
Microbiological Analysis of Tomatoes Coated with Pullulan Coating with EEP
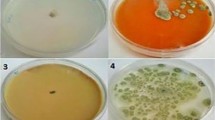
The effect of pullulan coating with EEP on antimicrobial activity was evaluated in artificially inoculated cherry tomatoes by estimating the reduction in the number of L. monocytogenes, S. Typhimurium, and E. coli bacteria during storage at 10 °C for 21 days (Fig. 2).
Coating of cherry tomatoes with P, P + 5% EEP, and P + 10% EEP resulted in a statistically significant reduction (p < 0.05) of S. Typhimurium, L. monocytogenes, and E. coli in relation to the initial number of bacteria. The P + 5% EEP and P + 10% EEP coatings were more effective than the P coating. The antimicrobial activity of these 2 coatings on tomatoes was found to be prolonged in the case of S. Typhimurium and L. monocytogenes. A reduction of 3.04 and 3.14 log CFU g−1 in the number of S. Typhimurium was on tomatoes coated with P + 5% EEP and P + 10% EEP, respectively, whereas the number of bacteria was decreased only by 1.73 log CFU g−1 on tomatoes coated with P after 14 days. Similarly, the number of L. monocytogenes was reduced by 3.02 and 3.28 log CFU g−1 only on P + 5% EEP- and P + 10% EEP-coated tomatoes after 21 days. The P coating contributed to the reduction of 2.27 log CFU g−1 in the bacterial count. The number of E. coli bacteria decreased by 1.68 and 1.89 log CFU g−1 on P + 5% EEP- and P + 10% EEP-coated tomatoes after 7 days, and P-coated tomatoes had a lower number of 0.7 log CFU g−1.
The effect of P, P + 5% EEP, and P + 10% EEP pullulan coatings on the development of mold on cherry tomatoes inoculated with P. chrysogenum, F. solani, and B. cinerea and stored at 10 °C for 21 days is shown in Fig. 2. A statistically significant decrease (p < 0.05) in the number of P. chrysogenum and F. solani cells on P-, P + 5% EEP-, and P + 10% EEP-coated tomatoes was observed on the first 7 days of storage in relation to the initial number of molds. The P + 5% EEP and P + 10% EEP coatings were more effective in reducing the number of molds than the P coating. The highest statistically significant decrease of about 1.9–2.2 log CFU g−1 (p < 0.05) in the number of P. chrysogenum and F. solani occurred after 7 days on P + 5% EEP- and P + 10% EEP-coated tomatoes. The P coating decreased the number of P. chrysogenum and F. solani by only about 1 log CFU g−1. After 14 and 21 days, no statistically significant decrease (p > 0.05) in the number of molds on the coated tomatoes was observed. All the 3 types of coatings did not inhibit the growth of B. cinerea.
In conclusion, the bacteriostatic and fungistatic activity of the pullulan coating containing propolis extract was observed on the coated tomatoes during the first 7 days of storage (reduction of microorganisms < 3 log CFU g−1). However, after 14 days, the P + 5% EEP and P + 10% EEP coatings were already found to be bactericidal (reduction of microorganisms > 3 log CFU g−1). The inclusion of EEP in the pullulan coating prolonged and enhanced its antibacterial activity. By contrast, no such phenomenon was observed for molds, the number of which was observed to decrease only up to the 7th day of storage. The fungicidal activity was only observed in the case of F. solani.
Stronger antimicrobial effect on tomatoes was achieved by using a combination of different treatments or coatings with a chemical preservative or a mixture of a natural antimicrobial substance and a chemical preservative, as evidenced by the studies of other authors. A combined treatment method involving mixed organic acid wash and use of chitosan-allyl isothiocyanate coating was shown to reduce the number of S. enterica by 7 log CFU g−1 and yeast and mold by more than 2 log CFU g−1 after 1 day of storage (Sudarsan Mukhopadhyay et al. 2018). A good antimold activity on tomatoes was also observed with coatings prepared from pea starch, potato starch, and guar gum with potassium sorbate (Mehyar et al. 2011) and coatings based on hydroxypropyl methylcellulose with beeswax containing sodium benzoate and other food additives (Fagundes et al. 2015; Fagundes et al. 2013). Research conducted by Ali et al. (2014) proved the antifungal efficacy of 5% propolis extract added to edible gum Arabic coatings on chili. Their results confirmed that EEP caused a gradual inhibition of mycelium growth within 14 days. About 0.5 to 1.0 cm decrease in the diameter of B. cinerea growth was observed on melatonin-coated cherry tomatoes (Li et al. 2019). A cassava starch–chitosan edible coating enriched with Lippia sidoides essential oil and pomegranate peel extract did not affect the reduction of mold and yeast counts on Italian tomatoes (Araújo et al. 2018).
Effect of Pullulan Coating with EEP on Changes in Total Soluble Solids, Titratable Acidity, Maturity Index, pH, Weight Loss, and Color in Tomatoes
TSS refers mainly to soluble sugars and is a very important indicator for assessing the quality of fruit and vegetables and determining consumer acceptability (Niu et al. 2019). The initial TSS content of cherry tomatoes was found to be 5.2% (Table 4). After 7 days of storage, it was significantly reduced (p < 0.05) to 4.8% and after 21 days to 4.5%. The type of coating as well as the storage time had no influence on the TSS values. The pH values of control tomatoes did not change during storage and were in the range of 4.65–4.59. Similarly, the type of coating and storage time had no significant effect (p > 0.05) on the pH of the tomatoes. With respect to TA, a significant effect of storage time was observed only in the case of uncoated and P-coated tomatoes, the values of which decreased significantly (p < 0.05) after 7, 14, and 21 days. The P + 5% EEP coating caused a statistically significant decrease (p < 0.05) in the TA values of tomatoes only after 7 days, while the P + 10% EEP coating did not affect this value during storage in the refrigerator. The MI (TSS-to-TA ratio) of control cherry tomatoes increased from 1.10 to 1.24. The P-coated cherry tomatoes showed an increase in the MI value from 1.16 to 1.20 during 21 days of storage, whereas the P + 5% EEP- and P + 10% EEP-coated tomatoes did not show any change during storage. According to Niu et al. (2019), the reduction in TSS can be caused by the hydrolysis of sucrose for respiration and maintaining the physiological activity of plant raw materials. Their study showed that pullulan ester film limited the reduction of TSS in strawberries compared with the control fruit. A lower decrease in the TA of P + 5% EEP- and P + 10% EEP-coated tomatoes compared with control tomatoes indicates a delay in the ripening of coated tomatoes, which is consistent with the studies of other authors (Medeiros et al. 2012; Salas-Méndez et al. 2019).
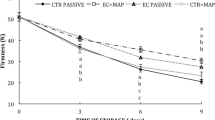
The loss of weight shortens the postharvest life of fresh fruit and vegetables. Figure 3 shows the changes in the weight of tomatoes during storage for 21 days. After 7 days, the weight loss of cherry tomatoes was found to be between 5.01 and 8.02%, and after 21 days, the loss increased from 11.03 to 20.73%. Such high weight loss in unpacked cherry tomatoes was caused by storage at an RH of ~ 60%. Similarly, weight losses of 10% and 19% were found by other authors when the samples were stored at 5 °C and 20 °C at an RH of 60% and 85% for 23 and 15 days, respectively (D’Aquino et al. 2016; C. Fagundes et al. 2015; Salas-Méndez et al. 2019).
The influence of the type of coating and storage time on the changes in the weight of cherry tomatoes was also investigated. The weight loss (%) during the storage of uncoated cherry tomatoes was statistically significantly (p < 0.05) higher than that of the coated cherry tomatoes. The P-coated tomatoes showed a statistically significantly (p < 0.05) lower weight loss compared with the uncoated tomatoes. The higher percentage of weight loss observed in the uncoated cherry tomatoes can be attributed to faster respiration and transpiration (Javanmardi and Kubota 2006). Thus, the pullulan coating itself acts as a barrier to water on the surface of fresh cherry tomatoes. Our results are consistent with those of Niu et al. (2019), who found that the weight loss of strawberries coated with pullulan ester film was lower than that of the uncoated strawberries. The authors reported that pullulan ester film formed a semipermeable barrier against oxygen, carbon dioxide, and moisture, and thus reduced the respiration, water loss, and oxidation. Similarly, an edible pectin- and corn flour-based coating was shown to reduce weight loss in tomatoes during storage (Sucheta et al. 2019). In turn, the use of rice bran wax coating reduced the weight loss of cherry tomatoes by only about 2% (Zhang et al. 2017). In addition, the present study showed that the addition of EEP to the pullulan coating further enhanced its action and reduced weight loss. Tomatoes coated with P + 10% EEP coating were characterized by a statistically significantly (p < 0.05) lower weight loss in comparison with the P- and P + 5% EEP-coated tomatoes. These observations are in agreement with the results of Salas-Méndez et al. (2019), who stated that the inclusion of leaf extract of Flourensia cernua in the nanolaminate-based coating delayed the weight loss of tomatoes compared with the nanolaminate-based coating without the extract. A weight loss of more than 5% reduces the postharvest life of fruit crops (Aktas et al. 2012). According to our estimates, the pullulan coating with propolis extract extended the postharvest life of cherry tomatoes by 3 days compared with the uncoated tomatoes and by 2 days compared with the P-coated ones.
Color is one of the criteria for choosing food products by consumers. The total color change above 5 is visible to the consumer. The results are shown in Fig. 4 relative to the uncoated sample of the week. P-coated tomatoes do not show a clear change in color compared with the control over the entire storage period. After 7 days of storage, P + 5% EEP- and P + 10% EEP-coated tomatoes do not differ significantly in color, but the ΔE parameter is greater than 5, which shows that tomatoes with these coatings are darker than the control.
Changes of tomato color during the storage period: coated tomatoes with pullulan coating (P), pullulan coating containing 5% propolis extract (P + 5% EEP), and pullulan coating containing 10% propolis extract (P + 10% EEP). Different superscript letters within the same days indicate significant (p < 0.05) differences of means. The mean values were compared using one-way analysis of variance using the Tukey test
The darker color of tomatoes indicates their antioxidant properties and higher lycopene content and determines their acceptability by consumers (Colonna et al. 2016; Erba et al. 2013). Coatings with antimicrobial additives may affect the color of the coated product (Kraśniewska et al. 2019). The use of plant molecule extracts and chitosan-allyl isothiocyanate coating did not change the color of tomatoes during storage (Mattson et al. 2011; Sudarsan Mukhopadhyay et al. 2018).
Sensory Evaluation
Sensory analysis was carried out within 2 days of tomato coating, as very high differences were noticed during the evaluation of traits in the first few days of coating in the study by Del-Valle et al. (2005). The sensory characteristics of uncoated and coated cherry tomatoes are shown in Fig. 5 in the form of a radar chart. In general, coated and uncoated cherry tomatoes were ranked almost equally for sensory properties, but coated tomatoes were found to have a better peel color. Coated tomatoes had a higher skin brightness than the uncoated ones, which may be due to the pullulan coating. The addition of EEP to the P coating did not eliminate the brightness of tomato peel. Moreover, the evaluators found no foreign color on P-, P + 5% EEP-, and P + 10% EEP-coated tomatoes in relation to the control samples. This proves that the addition of propolis extract to the pullulan coating caused no change in the color of the coated tomatoes. In addition, no significant statistical differences (p < 0.05) were found in the aroma and flavor of the tomatoes coated with P, P + 5% EEP, and P + 10% EEP in comparison with the control ones, and thus, it can be stated that propolis extract and pullulan coating did not change these sensory characteristics of cherry tomatoes. The overall quality of the coated tomatoes was also evaluated to be very high, which may result in high consumer acceptability. Due to their sharp, characteristic aroma and flavor, the use of propolis and EEP is limited in the food industry (Pobiega et al. 2019b). However, our study showed that the inclusion of propolis extract in the pullulan coating allows limiting the sensory properties of propolis. Similar observations were reported in the study by Pastor et al. (2011), in which grapes coated with a hydroxymethylcellulose coating containing propolis extract exhibited significantly better sensory properties than fruit coated with propolis extract alone.
Conclusions
In this study, it was found that EEP-containing pullulan coating on cherry tomatoes reduced the number of microorganisms and delayed ripening, which contributes to the extension of their shelf life. Pullulan coating containing EEP has significantly improved the chemical properties of cherry tomatoes during refrigerated storage. Furthermore, the addition of EEP to the pullulan coating did not affect the flavor and aroma of tomatoes, while increasing the brightness of the skin, making coated cherry tomatoes more acceptable to evaluators.
Change history
21 July 2020
The original version of this article unfortunately had typo error in one of the author���s name.
References
Ahmad, A., Kaleem, M., Ahmed, Z., & Shafiq, H. (2015). Therapeutic potential of flavonoids and their mechanism of action against microbial and viral infections-A review. Food Research International, 77, 221–235. https://doi.org/10.1016/j.foodres.2015.06.021.
Aktas, H., Bayındır, D., Dilmaçünal, T., & Koyuncu, M. A. (2012). The effects of minerals, ascorbic acid, and salicylic acid on the bunch quality of tomatoes (Solanum lycopersicum) at high and low temperatures. HortScience, 47(10), 1478–1483.
Ali, A., Cheong, C. K., & Zahid, N. (2014). Composite effect of propolis and gum arabic to control postharvest anthracnose and maintain quality of papaya during storage. International Journal of Agriculture and Biotechnology, 16(6), 1117–1122.
Araújo, J. M. S., de Siqueira, A. C. P., Blank, A. F., Narain, N., & de Aquino Santana, L. C. L. (2018). A cassava starch–chitosan edible coating enriched with Lippia sidoides Cham. essential oil and pomegranate peel extract for preservation of Italian tomatoes (Lycopersicon esculentum Mill.) stored at room temperature. Food and Bioprocess Technology, 11(9), 1750–1760. https://doi.org/10.1007/s11947-018-2139-9.
Asawahame, C., Sutjarittangtham, K., Eitssayeam, S., Tragoolpua, Y., Sirithunyalug, B., & Sirithunyalug, J. (2015). Antibacterial activity and inhibition of adherence of Streptococcus mutans by propolis electrospun fibers. AAPS PharmSciTech, 16(1), 182–191. https://doi.org/10.1208/s12249-014-0209-5.
Asensio, E., Sanvicente, I., Mallor, C., & Menal-Puey, S. (2019). Spanish traditional tomato. Effects of genotype, location and agronomic conditions on the nutritional quality and evaluation of consumer preferences. Food Chemistry, 270, 452–458. https://doi.org/10.1016/j.foodchem.2018.07.131.
Balouiri, M., Sadiki, M., & Ibnsouda, S. K. (2016). Methods for in vitro evaluating antimicrobial activity: A review. Journal of Pharmaceutical Analysis. Xi’an Jiaotong University, 6(2), 71–79. https://doi.org/10.1016/j.jpha.2015.11.005.
Ban, G. H., & Kang, D. H. (2018). Inactivation of Escherichia coli O157:H7, Salmonella typhimurium, and Listeria monocytogenes on cherry tomatoes and oranges by superheated steam. Food Research International, 112, 38–47. https://doi.org/10.1016/j.foodres.2018.05.069.
Bankova, V. (2005). Chemical diversity of propolis and the problem of standardization. Journal of Ethnopharmacology, 100(1-2), 114–117. https://doi.org/10.1016/j.jep.2005.05.004.
Cabrera-Díaz, E., Martínez-Chávez, L., Sánchez-Camarena, J., Muñiz-Flores, J. A., Castillo, A., Gutiérrez-González, P., Arvizu-Medrano, S. M., González-Aguilar, D. G., & Martínez-Gonzáles, N. E. (2018). Simultaneous and individual quantitative estimation of Salmonella, Shigella and Listeria monocytogenes on inoculated Roma tomatoes (Lycopersicon esculentum var. pyriforme) and Serrano peppers (Capsicum annuum) using an MPN technique. Food Microbiology, 73, 282–287. https://doi.org/10.1016/j.fm.2018.02.009.
Chang, A. S., & Schneider, K. R. (2012). Evaluation of overhead spray-applied sanitizers for the reduction of Salmonella on tomato surfaces. Journal of Food Science, 77(1), M65–M69. https://doi.org/10.1111/j.1750-3841.2011.02486.x.
Chen, H., & Zhong, Q. (2018). Antibacterial activity of acidified sodium benzoate against Escherichia coli O157:H7, Salmonella enterica, and Listeria monocytogenes in tryptic soy broth and on cherry tomatoes. International Journal of Food Microbiology, 274, 38–44. https://doi.org/10.1016/j.ijfoodmicro.2018.03.017.
Chen, Y. W., Ye, S. R., Ting, C., & Yu, Y. H. (2018). Antibacterial activity of propolins from Taiwanese green propolis. Journal of Food and Drug Analysis, 26(2), 761–768. https://doi.org/10.1016/j.jfda.2017.10.002.
Cheng, K. C., Demirci, A., & Catchmark, J. M. (2011). Pullulan: Biosynthesis, production, and applications. Applied Microbiology and Biotechnology., 92(1), 29–44. https://doi.org/10.1007/s00253-011-3477-y.
Colonna, E., Rouphael, Y., Barbieri, G., & De Pascale, S. (2016). Nutritional quality of ten leafy vegetables harvested at two light intensities. Food Chemistry, 199, 702–710. https://doi.org/10.1016/j.foodchem.2015.12.068.
D’Aquino, S., Mistriotis, A., Briassoulis, D., Di Lorenzo, M. L., Malinconico, M., & Palma, A. (2016). Influence of modified atmosphere packaging on postharvest quality of cherry tomatoes held at 20°C. Postharvest Biology and Technology, 115, 103–112. https://doi.org/10.1016/j.postharvbio.2015.12.014.
De Araújo, G. K. P., De Souza, S. J., Da Silva, M. V., Yamashita, F., Gonçalves, O. H., Leimann, F. V., & Shirai, M. A. (2015). Physical, antimicrobial and antioxidant properties of starch-based film containing ethanolic propolis extract. International Journal of Food Science and Technology, 50(9), 2080–2087. https://doi.org/10.1111/ijfs.12869.
de Groot, A. C., Popova, M. P., & Bankova, V. S. (2014). An update on the constituents of poplar-type propolis (pp. 1–11). Wapserveen: Acdegroot Publishing.
Del-Valle, V., Hernández-Muñoz, P., Guarda, A., & Galotto, M. J. (2005). Development of a cactus-mucilage edible coating (Opuntia ficus indica) and its application to extend strawberry (Fragaria ananassa) shelf-life. Food Chemistry, 91(4), 751–756. https://doi.org/10.1016/j.foodchem.2004.07.002.
Erba, D., Casiraghi, M. C., Ribas-Agustí, A., Cáceres, R., Marfà, O., & Castellari, M. (2013). Nutritional value of tomatoes (Solanum lycopersicum L.) grown in greenhouse by different agronomic techniques. Journal of Food Composition and Analysis, 31(2), 245–251. https://doi.org/10.1016/j.jfca.2013.05.014.
Fagundes, C., Pérez-Gago, M. B., Monteiro, A. R., & Palou, L. (2013). Antifungal activity of food additives in vitro and as ingredients of hydroxypropyl methylcellulose-lipid edible coatings against Botrytis cinerea and Alternaria alternata on cherry tomato fruit. International Journal of Food Microbiology, 166(3), 391–398. https://doi.org/10.1016/j.ijfoodmicro.2013.08.001.
Fagundes, C., Moraes, K., Pérez-Gago, M. B., Palou, L., Maraschin, M., & Monteiro, A. R. (2015). Effect of active modified atmosphere and cold storage on the postharvest quality of cherry tomatoes. Postharvest Biology and Technology, 109, 73–81. https://doi.org/10.1016/j.postharvbio.2015.05.017.
Galletti, J., Tobaldini-Valerio, F. K., Silva, S., Kioshima, É. S., Trierveiler-Pereira, L., Bruschi, M., Negri, M., & Estivalet Svidzinski, T. I. (2017). Antibiofilm activity of propolis extract on Fusarium species from onychomycosis. Future Microbiology, 12(14), 1311–1321. https://doi.org/10.2217/fmb-2017-0052.
Gniewosz, M., & Duszkiewicz-Reinhard, W. (2008). Comparative studies on pullulan synthesis, melanin synthesis and morphology of white mutant Aureobasidium pullulans B-1 and parent strain A.p.-3. Carbohydrate Polymers, 72(3), 431–438. https://doi.org/10.1016/J.CARBPOL.2007.09.009.
Gniewosz, M., Kraśniewska, K., Woreta, M., & Kosakowska, O. (2013). Antimicrobial activity of a pullulan-caraway essential oil coating on reduction of food microorganisms and quality in fresh baby carrot. Journal of Food Science, 78(8), M1242–M1248. https://doi.org/10.1111/1750-3841.12217.
Graikou, K., Popova, M., Gortzi, O., Bankova, V., & Chinou, I. (2016). Characterization and biological evaluation of selected Mediterranean propolis samples. Is it a new type? LWT - Food Science and Technology, 65, 261–267. https://doi.org/10.1016/j.lwt.2015.08.025.
Grecka, K., Kuś, P. M., Okińczyc, P., Worobo, R. W., Walkusz, J., Szweda, P., et al. (2019). The anti-staphylococcal potential of ethanolic Polish propolis extracts. Molecules, 24(9). https://doi.org/10.3390/molecules24091732.
Gündüz, G. T., Gönül, S. A., & Karapinar, M. (2010). Efficacy of sumac and oregano in the inactivation of Salmonella Typhimurium on tomatoes. International Journal of Food Microbiology, 141(1–2), 39–44. https://doi.org/10.1016/j.ijfoodmicro.2010.04.021.
Guo, H., Qin, X., Wu, Y., Yu, W., Liu, J., Xi, Y., Dou, G., Wang, L., & Xiao, H. (2019). Biocontrol of gray mold of cherry tomatoes with the volatile organic monomer from Hanseniaspora uvarum, trans -cinnamaldehyde. Food and Bioprocess Technology, 20(11), 1809–1820. https://doi.org/10.1007/s11947-019-02319-6.
Isidorov, V. A., Szczepaniak, L., & Bakier, S. (2014). Rapid GC/MS determination of botanical precursors of Eurasian propolis. Food Chemistry, 142, 101–106. https://doi.org/10.1016/j.foodchem.2013.07.032.
Jansen-Alves, C., Maia, D. S. V., Krumreich, F. D., Crizel-Cardoso, M. M., Fioravante, J. B., da Silva, W. P., Borges, C. D., & Zambiazi, R. C. (2019). Propolis microparticles produced with pea protein: Characterization and evaluation of antioxidant and antimicrobial activities. Food Hydrocolloids, 87, 703–711. https://doi.org/10.1016/j.foodhyd.2018.09.004.
Javanmardi, J., & Kubota, C. (2006). Variation of lycopene, antioxidant activity, total soluble solids and weight loss of tomato during postharvest storage. Postharvest Biology and Technology, 41(2), 151–155. https://doi.org/10.1016/j.postharvbio.2006.03.008.
Kayode, R. M., Azubuike, C. U., Laba, S. A., Dauda, A. O., Balogun, M. A., & Ajala, S. A. (2018). Chemical composition and anti-microbial activities of the essential oil of Adansonia digitata stem-bark and leaf on post-harvest control of tomato spoilage. LWT, 93, 58–63. https://doi.org/10.1016/j.lwt.2018.03.014.
Kraśniewska, K., Gniewosz, M., Kosakowska, O., & Cis, A. (2016). Preservation of Brussels sprouts by pullulan coating containing oregano essential oil. Journal of Food Protection, 79(3), 493–500. https://doi.org/10.4315/0362-028X.JFP-15-234.
Kraśniewska, K., Pobiega, K., & Gniewosz, M. (2019). Pullulan-biopolymer with potential for use as food packaging. International Journal of Food Engineering, 15(9), 20190030. https://doi.org/10.1515/ijfe-2019-0030.
Kuś, P. M., Okińczyc, P., Jakovljević, M., Jokić, S., & Jerković, I. (2018). Development of supercritical CO2 extraction of bioactive phytochemicals from black poplar (Populus nigra L.) buds followed by GC–MS and UHPLC-DAD-QqTOF-MS. Journal of Pharmaceutical and Biomedical Analysis, 158, 15–27. https://doi.org/10.1016/J.JPBA.2018.05.041.
Li, S., Xu, Y., Bi, Y., Zhang, B., Shen, S., Jiang, T., & Zheng, X. (2019). Melatonin treatment inhibits gray mold and induces disease resistance in cherry tomato fruit during postharvest. Postharvest Biology and Technology, 157, 110962. https://doi.org/10.1016/j.postharvbio.2019.110962.
Lin, F., Xue, Y., Huang, Z., Jiang, M., Lu, F., Bie, X., Miao, S., & Lu, Z. (2019). Bacillomycin D inhibits growth of Rhizopus stolonifer and induces defense-related mechanism in cherry tomato. Applied Microbiology and Biotechnology, 103(18), 7663–7674. https://doi.org/10.1007/s00253-019-09991-w.
Lu, Y., Joerger, R., & Wu, C. (2014). Similar reduction of Salmonella enterica Typhimurium on grape tomatoes and its cross-contamination in wash water by washing with natural antimicrobials as compared with chlorine treatment. Food and Bioprocess Technology, 7(3), 661–670. https://doi.org/10.1007/s11947-013-1105-9.
Ma, Z. C., Fu, W. J., Liu, G. L., Wang, Z. P., & Chi, Z. M. (2014). High-level pullulan production by Aureobasidium pullulans var. melanogenium P16 isolated from mangrove system. Applied Microbiology and Biotechnology, 98(11), 4865–4873. https://doi.org/10.1007/s00253-014-5554-5.
Machado, C. S., Mokochinski, J. B., De Lira, T. O., Oliveira, F. D. C. D. E., Cardoso, M. V., Ferreira, R. G., et al. (2016). Comparative study of chemical composition and biological activity of yellow, green, brown, and red Brazilian propolis. Evidence-based Complementary and Alternative Medicine, 2016, 6057650. https://doi.org/10.1155/2016/6057650.
Madureira, J., Severino, A., Cojocaru, M., Garofalide, S., Santos, P. M. P., Carolino, M. M., Margaça, F. M. A., & Cabo Verde, S. (2019). E-beam treatment to guarantee the safety and quality of cherry tomatoes. Innovative Food Science and Emerging Technologies, 55, 57–65. https://doi.org/10.1016/j.ifset.2019.05.013.
Mahieddine, B., Amina, B., Faouzi, S. M., Sana, B., & Wided, D. (2018). Effects of microwave heating on the antioxidant activities of tomato (Solanum lycopersicum). Annals of Agricultural Sciences, 63(2), 135–139. https://doi.org/10.1016/j.aoas.2018.09.001.
Mascheroni, E., Guillard, V., Nalin, F., Mora, L., & Piergiovanni, L. (2010). Diffusivity of propolis compounds in polylactic acid polymer for the development of anti-microbial packaging films. Journal of Food Engineering, 98(3), 294–301. https://doi.org/10.1016/j.jfoodeng.2009.12.028.
Mascheroni, E., Figoli, A., Musatti, A., Limbo, S., Drioli, E., Suevo, R., Talarico, S., & Rollini, M. (2014). An alternative encapsulation approach for production of active chitosan-propolis beads. International Journal of Food Science & Technology, 49(5), 1401–1407. https://doi.org/10.1111/ijfs.12442.
Mattson, T. E., Johny, A. K., Amalaradjou, M. A. R., More, K., Schreiber, D. T., Patel, J., & Venkitanarayanan, K. (2011). Inactivation of Salmonella spp. on tomatoes by plant molecules. International Journal of Food Microbiology, 144(3), 464–468. https://doi.org/10.1016/j.ijfoodmicro.2010.10.035.
Medeiros, B. G. d. S., Pinheiro, A. C., Teixeira, J. A., Vicente, A. A., & Carneiro-da-Cunha, M. G. (2012). Polysaccharide/protein nanomultilayer coatings: Construction, characterization and evaluation of their effect on “Rocha” pear (Pyrus communis L.) shelf-life. Food and Bioprocess Technology, 5(6), 2435–2445. https://doi.org/10.1007/s11947-010-0508-0.
Mehyar, G. F., Al-Qadiri, H. M., Abu-Blan, H. A., & Swanson, B. G. (2011). Antifungal effectiveness of potassium sorbate incorporated in edible coatings against spoilage molds of apples, cucumbers, and tomatoes during refrigerated storage. Journal of Food Science, 76(3), M210–M217. https://doi.org/10.1111/j.1750-3841.2011.02059.x.
Mohdaly, A. A. A., Mahmoud, A. A., Roby, M. H. H., Smetanska, I., & Ramadan, M. F. (2015). Phenolic extract from propolis and bee pollen: Composition, antioxidant and antibacterial activities. Journal of Food Biochemistry, 39(5), 538–547. https://doi.org/10.1111/jfbc.12160.
Morsy, M. K., Khalaf, H. H., Sharoba, A. M., El-Tanahi, H. H., & Cutter, C. N. (2014). Incorporation of essential oils and nanoparticles in pullulan films to control foodborne pathogens on meat and poultry products. Journal of Food Science, 79(4), M675–M684. https://doi.org/10.1111/1750-3841.12400.
Mukhopadhyay, S., Ukuku, D., Fan, X., & Juneja, V. K. (2013). Efficacy of integrated treatment of UV light and low-dose gamma irradiation on inactivation of Escherichia coli O157:H7 and Salmonella enterica on grape tomatoes. Journal of Food Science, 78(7), M1049–M1056. https://doi.org/10.1111/1750-3841.12154.
Mukhopadhyay, S., Sokorai, K., Ukuku, D. O., Jin, T., Fan, X., Olanya, M., & Juneja, V. (2018). Inactivation of Salmonella in grape tomato stem scars by organic acid wash and chitosan-allyl isothiocyanate coating. International Journal of Food Microbiology, 266, 234–240. https://doi.org/10.1016/j.ijfoodmicro.2017.12.018.
Niu, B., Shao, P., Chen, H., & Sun, P. (2019). Structural and physiochemical characterization of novel hydrophobic packaging films based on pullulan derivatives for fruits preservation. Carbohydrate Polymers, 208, 276–284. https://doi.org/10.1016/j.carbpol.2018.12.070.
Pastor, C., Sanchez-Gonzalez, L., Marcilla, A., Chiralt, A., Cháfer, M., & Gonzalez-Martinez, C. (2011). Quality and safety of table grapes coated with hydroxypropylmethylcellulose edible coatings containing propolis extract. Postharvest Biology and Technology, 60(1), 64–70. https://doi.org/10.1016/j.postharvbio.2010.11.003.
Pobiega, K., Gniewosz, M., & Kraśniewska, K. (2017). Antimicrobial and antiviral properties of different types of propolis. Zeszyty Problemowe Postępów Nauk Rolniczych, 589, 69–79. https://doi.org/10.22630/ZPPNR.2017.589.22.
Pobiega, K., Kraśniewska, K., Derewiaka, D., & Gniewosz, M. (2019a). Comparison of the antimicrobial activity of propolis extracts obtained by means of various extraction methods. Journal of Food Science and Technology, 56(12), 5386–5395. https://doi.org/10.1007/s13197-019-04009-9.
Pobiega, K., Kraśniewska, K., & Gniewosz, M. (2019b). Application of propolis in antimicrobial and antioxidative protection of food quality – A review. Trends in Food Science & Technology, 83, 53–62. https://doi.org/10.1016/J.TIFS.2018.11.007.
Pobiega, K., Kraśniewska, K., Przybył, J. L., Bączek, K., Żubernik, J., Witrowa-Rajchert, D., & Gniewosz, M. (2019c). Growth biocontrol of foodborne pathogens and spoilage microorganisms of food by Polish propolis extracts. Molecules, 24(16), 2965. https://doi.org/10.3390/molecules24162965.
Popova, M., Chen, C.-N., Chen, P.-Y., Huang, C.-Y., & Bankova, V. (2010). A validated spectrophotometric method for quantification of prenylated flavanones in Pacific propolis from Taiwan. Phytochemical Analysis, 21, 186–191. https://doi.org/10.1002/pca.1176.
Popova, M., Giannopoulou, E., Skalicka-Woźniak, K., Graikou, K., Widelski, J., Bankova, V., Kalofonos, H., Sivolapenko, G., Gaweł-Bęben, K., Antosiewicz, B., & Chinou, I. (2017). Characterization and biological evaluation of propolis from Poland. Molecules, 22(7), 1159. https://doi.org/10.3390/molecules22071159.
Przybyłek, I., & Karpiński, T. M. (2019). Antibacterial properties of propolis. Molecules, 24(11), 2047. https://doi.org/10.3390/molecules24112047.
Rekha, M., & Sharma, C. P. (2007). Pullulan as a promising biomaterial for biomedical applications: A perspective. Biomaterials and Artificial Organs, 20(2), 116–121.
Ripari, V., Bai, Y., & Gänzle, M. G. (2019). Metabolism of phenolic acids in whole wheat and rye malt sourdoughs. Food Microbiology, 77, 43–51. https://doi.org/10.1016/j.fm.2018.08.009.
Salas-Méndez, E. d. J., Vicente, A., Pinheiro, A. C., Ballesteros, L. F., Silva, P., Rodríguez-García, R., et al. (2019). Application of edible nanolaminate coatings with antimicrobial extract of Flourensia cernua to extend the shelf-life of tomato (Solanum lycopersicum L.) fruit. Postharvest Biology and Technology, 150, 19–27. https://doi.org/10.1016/j.postharvbio.2018.12.008.
Standards Institute Clinical Laboratory (CLSI). (2006). Performance standards for antimicro- bial disk susceptibility tests; Approved standard—9th ed. CLSI document M2-A9. Wayne, PA: Clinical Laboratory Standards Institute, 26(1).
Sucheta, Chaturvedi, K., Sharma, N., & Yadav, S. K. (2019). Composite edible coatings from commercial pectin, corn flour and beetroot powder minimize post-harvest decay, reduces ripening and improves sensory liking of tomatoes. International Journal of Biological Macromolecules, 133, 284–293. https://doi.org/10.1016/j.ijbiomac.2019.04.132.
Synowiec, A., Gniewosz, M., Kraśniewska, K., Chlebowska-Śmigiel, A., Przybył, J. L., Bączek, K., & Węglarz, Z. (2014a). Effect of meadowsweet flower extract-pullulan coatings on Rhizopus rot development and postharvest quality of cold-stored red peppers. Molecules, 19(9), 12925–12939. https://doi.org/10.3390/molecules190912925.
Synowiec, A., Gniewosz, M., Kraśniewska, K., Przybył, J. L., Bączek, K., & Węglarz, Z. (2014b). Antimicrobial and antioxidant properties of pullulan film containing sweet basil extract and an evaluation of coating effectiveness in the prolongation of the shelf life of apples stored in refrigeration conditions. Innovative Food Science and Emerging Technologies, 23, 171–181. https://doi.org/10.1016/j.ifset.2014.03.006.
Tang, Q., Zhu, F., Cao, X., Zheng, X., Yu, T., & Lu, L. (2019). Cryptococcus laurentii controls gray mold of cherry tomato fruit via modulation of ethylene-associated immune responses. Food Chemistry, 278, 240–247. https://doi.org/10.1016/j.foodchem.2018.11.051.
Terzopoulos, P. J., & Bebeli, P. J. (2010). Phenotypic diversity in Greek tomato (Solanum lycopersicum L.) landraces. Scientia Horticulturae, 126(2), 138–144. https://doi.org/10.1016/j.scienta.2010.06.022.
Torlak, E., & Sert, D. (2013). Antibacterial effectiveness of chitosan-propolis coated polypropylene films against foodborne pathogens. International Journal of Biological Macromolecules, 60, 52–55. https://doi.org/10.1016/j.ijbiomac.2013.05.013.
Valdivia-Nájar, C. G., Martín-Belloso, O., Giner-Seguí, J., & Soliva-Fortuny, R. (2017). Modeling the inactivation of Listeria innocua and Escherichia coli in fresh-cut tomato treated with pulsed light. Food and Bioprocess Technology, 10(2), 266–274. https://doi.org/10.1007/s11947-016-1806-y.
Wolska, K., Górska, A., & Adamiak, A. (2016). Właściwości przeciwbakteryjne propolisu. Postępy Mikrobiologii, 55(4), 343–350.
Yang, H., Huang, Z., Chen, Y., Zhang, C., Ye, M., & Wang, L. (2016). Evaluation of the contributions of polyphenols in Chinese propolis by on-line HPLC–ABTS method. European Food Research and Technology, 242(4), 537–546. https://doi.org/10.1007/s00217-015-2564-1.
Yuk, H.-G., Bartz, J. A., & Schneider, K. R. (2006). Effectiveness of individual or combined sanitizer treatments for inactivating Salmonella spp. on smooth surface, stem scar, and wounds of tomatoes. Journal of Food Science, 70(9), M409–M414. https://doi.org/10.1111/j.1365-2621.2005.tb08326.x.
Zargaran, M., Taghipour, S., Kiasat, N., Aboualigalehdari, E., Rezaei-Matehkolaei, A., Zarei Mahmoudabadi, A., & Shamsizadeh, F. (2017). Luliconazole, an alternative antifungal agent against Aspergillus terreus. Journal de Mycologie Medicale, 27(3), 351–356. https://doi.org/10.1016/j.mycmed.2017.04.011.
Zhang, L., Chen, F., Zhang, P., Lai, S., & Yang, H. (2017). Influence of rice bran wax coating on the physicochemical properties and pectin nanostructure of cherry tomatoes. Food and Bioprocess Technology, 10(2), 349–357. https://doi.org/10.1007/s11947-016-1820-0.
Zhou, B., Luo, Y., Bauchan, G. R., Feng, H., & Stommel, J. R. (2018). Visualizing pathogen internalization pathways in fresh tomatoes using MicroCT and confocal laser scanning microscopy. Food Control, 85, 276–282. https://doi.org/10.1016/j.foodcont.2017.09.027.
Acknowledgments
The authors thank Translmed (Cedar Hill, TX, USA), a proofreading and copyediting company, for helping in copyediting this manuscript.
Funding
This study was supported by the grant from the Polish Ministry of Science and Higher Education for the implementation of the project for young scientists (Grant No. 505-10-092800-P00210-99).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Pobiega, K., Przybył, J.L., Żubernik, J. et al. Prolonging the Shelf Life of Cherry Tomatoes by Pullulan Coating with Ethanol Extract of Propolis During Refrigerated Storage. Food Bioprocess Technol 13, 1447–1461 (2020). https://doi.org/10.1007/s11947-020-02487-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11947-020-02487-w