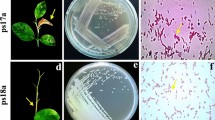
Abstract
One of the most common diseases affecting Stone-fruits is Bacterial Canker, caused by Pseudomonas syringae pv. syringae (Pss) and morsprunorum (Psm). In this study, Pss and Psm were isolated from stone-fruit trees from the Western Aegean region (WA) and Lake Van Basin (LVB) of Turkey, where pesticide usage is high and low, respectively. We aimed to determine copper resistance levels, the involved resistance mechanisms and the reactions to copper pesticides of these isolates. The minimum inhibitory concentration (MIC) and lethal dose 50 (LD50) of the isolates were detected using CuSO4 and other Cu-pesticides. To determine the mechanisms of copper resistance, the presence of copA and cusA genes was investigated. The modified Fe/Cu Blue-CAS Agar media were used to investigate the relationship between the isolates’ copper resistance and each isolate’s ability to produce siderophores. The highest MIC value was 2 mM in CuSO4. The tolerance levels of the isolates from the WA and LVB regions were 29% and 47% susceptible, 29% and 47% low resistance and 42% and 6% resistant, respectively. The most successful pesticide was (CuSO4 + Ca (OH)2) + mancozeb. While none of the isolates had the cusA gene, four isolates had the copA gene, which was proven to be plasmid-borne. Differences between copA gene sequences were detected and were determined to be not related to the pathovars. The amount of siderophore produced against copper in Ps pathovars affected seems to be related with the resistance level. Also, Ps pathovars were able to tolerate copper at doses as high as 1.1 mM by producing siderophores, and at doses of 1.7 mM and above through the copA gene.


Similar content being viewed by others
References
Agrios, G., (2005). Plant pathology. Fifty Ed. Ed.sl.: Elsiver Academic Pres, 922p.
Aiello, D., Ferrante, P., Vitale, A., Polizzi, G., Scortichini, M., & Cirvilleri, G. (2015). Characterization of Pseudomonas syringae pv. syringae isolated from mango in sicily and occurrence of copper-resistant strains. Plant Pathology, 97(2), 273–282.
Akköprü, A. (2016). Determination of bacterial disease on stone fruits grown in Lake Van Basin, East Anatolia of Turkey. ActaHortic, 1149, 15–20.
Altimira, F., Yá̃ez, C., Bravo, G., González, M., Rojas, L. A., & Seeger, M. (2012). Characterization of copper-resistant bacteria and bacterial communities from copper-polluted agricultural soils of Central Chile. BMC Microbiology, 12, 193–205.
Anonymous. (2017). Toplam tarım ilacı kullanımını. Çevre ve şehircilik bakanlığı, Türkiye. Erişim tarihi: 12.11.2018. https://cevreselgostergeler.csb.gov.tr/tarim-ilaci-pestisit-kullanimi-i-85834.
Argüello, J. M., Raimunda, D., & Padilla-Benavides, T. (2013). Mechanisms of copper homeostasis in bacteria. Frontiers in Cellular and Infection Microbiology, 3, 1–14.
Badar, U., Ahmed, N., Shoeb, E., & Gadd, G. M. (2014). Identification of the pco operon in Enterobacter species isolated from contaminated soil. International Journal of Advanced Research, 2(3), 227–233.
Behlau, F., Belasque Jr., J., Gragam, J., & Leite Jr., R. P. (2010). Effect of frequency of copper applications on control of citrus canker and the yield of young bearing sweet orange trees. Crop Protection, 29, 300–305.
Behlau, F., Gochez, M. A., Lugo, J. A., Elibox, W., Minsavage, V. G., Potnis, N., et al. (2017). Characterization of a unique copper resistance gene cluster in Xanthomonas campestris pv. campestris isolated in Trinidad, West Indies. European Journal of Plant Pathology, 147(3), 671–681.
Bender, C. L., & Cooksey, D. A. (1986). Indigenous plasmids in Pseudomonas syringae pv. tomato: Conjugative transfer and role in copper resistance. Journal of Bacteriology, 165(2), 534–541.
Benlioğlu, K., & Benlioğlu, S. (1998), Pseudomonas syringae pv. tomato’ya karşı bakır dayanıklılığı üzerinde çalışmalar, 8. Turkey Phytopathology Congress, 21–25 September 1998, Ankara, Turkey (in Turkish).
Besaury, L., Bodilis, J., Delgas, F., Andrade, S., De la Iglesia, R., Ouddane, B., & Quillet, L. (2013). Abundance and diversity of copper resistance genes cusA and copA in microbial communities in relation to the impact of copper on Chilean marine sediments. Marine Pollution Bulletin, 67(1–2), 16–25.
Bondarczuk, K., & Piotrowska-Seget, Z. (2013). Molecular basis of active copper resistance mechanisms in gram-negative bacteria. Cell Biology and Toxicology, 29(6), 397–405.
Braud, A., Geoffroy, V., Hoegy, F., Mislin, G., & Schalk, I. (2010). Presence of the siderophores pyoverdine and pyochelin in the extracellular medium reduces toxic metal accumulation in Pseudomonas aeruginosa andincreases bacterial metal tolerance. Environmental Microbiology, 2(3), 419–425.
Braud, A., Hoegy, F., Jezequel, K., Lebeau, T., & Schalk, I. J. (2009). New insights into the metal specificity of the Pseudomonas aeruginosa pyoverdine–iron uptake pathway. Environmental Microbiology, 11(5), 1079–1091.
Bultreys, A., & Kaluzna, M. (2010). Bacterial cankers caused by Pseudomonas syringae on stone fruit species with special emphasis on the pathovars syringae and morsprunorum race 1 and race 2. Journal of Plant Pathology, 92(1), 21–33.
Cazorla, F. M., Arrebola, E., Sesma, A., Pérez-García, A., Codina, J. C., Murillo, J., & Vicente, A. (2002). Copper resistance in Pseudomonas syringae strains isolated from mango is encoded mainly by plasmids. Phytopatholog, 92, 909–916.
Cha, J-S., & Cooksey, D. A. (1991). Copper resistance in Pseudomonas syringae mediated by periplasmic and outer membrane proteins. Proceedings of the National Academy of Sciences, 88(20), 8915–8919.
Chaturvedi, K. S., Hung, C. S., Crowley, J. R., Stapleton, A. E., & Henderson, J. P. (2012). The siderophore yersiniabactin binds copper to protect pathogens during infection. Nature Chemical Biology, 8(8), 731–736.
Conlin, K. C., & McCarter, S. M. (2008). Effectiveness of selected chemicals in inhibiting Pseudomonas syringae pv. tomato in-vitro and in controlling bacterial speck. Plant Disease, 67(6), 639–644.
Cooksey, D. A. (1987). Characterization of a copper resistance plasmid conserved in copper-resistant strains of Pseudomonas syringae pv. tomato. Applied and Environmental Microbiology, 53(2), 454–456.
Cooksey, D. A. (1990). Plasmid-determined copper resistance in Pseudomonas syringae from impatiens. Applied and Environmental Microbiology, 56(1), 13–16.
Cooksey, D. A., AZAD, H. R., Cha, J.-S., & Lim, C.-K. (1990). Copper resistance gene homologs in pathogenic and saprophytic bacterial species from tomato. Applied and Environmental Microbiology, 56(2), 431–435.
Cooksey, D. A., & Azad, H. R. (1992). Accumulation of copper and other metals by copper-resistant plant-pathogenic and saprophytic Pseudomonads. Applied and Environmental Microbiology, 58(1), 274–278.
Cornelis, P., & Matthijs, S. (2007). Pseudomonas Siderophores and their biological significance. In: Soil biology, volume 12 Microbial Siderophores A. Varma, S.B. Chincholkar (Eds.). Berlin Heidelberg: Springer-Verlag.
Donmez, M. F., Karlidag, H., & Esitken, A. (2010). Identification of resistance to bacterial canker (Pseudomonas syringae pv. syringae) disease on apricot genotypes grown in Turkey. Eur J Plant Patho, 126, 241–247.
Eğerci, K. (2015). Studies on the sensitivity level of some plant pathogenic and saprophytic bacteria against copper-based compounds. MSc in Department of Plant Protection. Ege University, Graduate School of Natural and Applied Science, Izmir, Turkey. (in Turkish).
FAO, 2017. http://faostat.fao.org/site/339/default.aspx.
Grass, G., & Rensing, C. (2001). Genes involved in copper homeostasis in Escherichia coli. Journal of Bacteriology, 183, 2145–2147.
Grass, G., Thakali, K., Klebba, P. E., Thieme, D., Muller, A., Wildner, G. F., & Rensing, C. (2004). Linkage between catecholate siderophores and the multicopper oxidase CueO in Escherichia coli. Journal of Bacteriology, 186, 5826–5833.
Gutiérrez-Barranquero, J. A., de Vicente, A., Carrion, V. J., Sundin, G. W., & Cazorla, F. M. (2013a). Recruitment and rearrangement of three different genetic determinants into a conjugative plasmid increase copper resistance in Pseudomonas syringae. Applied and Environmental Microbiology, 79(3), 1028–1033.
Gutiérrez-Barranquero, J. A., Carrión, V. J., Murillo, J., Arrebola, E., Arnold, D. L., Cazorla, F. M., & de Vicente, A. (2013b). A Pseudomonas syringae diversity survey reveals a differentiated phylotype of the pathovar syringae associated with the mango host and mangotoxin production. Phytopathology, 103(11), 1115–1129.
Hernández-Montes, G., Argüello, J. M., & Valderrama, B. (2012). Evolution and diversity of periplasmic proteins involved in copper homeostasis in gamma proteobacteria. BMC Microbiology, 12, 249–263.
Hirano, S. S., & Upper, C. D. (2000). Bacteria in the leaf ecosystem with emphasis on Pseudomonas syringae a pathogen, ice nucleus, and epiphyte. Microbiology and Molecular Biology Reviews. https://doi.org/10.1128/MMBR.64.3.624-653.2000.
Ivanović, Ž., Perović, T., Popović, T., Blagojević, J., Trkulja, N., & Hrnčić, S. (2017). Characterization of Pseudomonas syringae pv. syringae, causal agent of citrus blast of mandarin in Montenegro. Plant Pathol. J, 33(1), 21–33.
Janse, J. D. (2010). Diagnostic methods for phytopathogenic bacteria of stone fruits and nuts in COST 873. EPPO Bulletin, 40, 68–85.
Kałużna, M., Willems, A., Pothier, J. L. F., Ruinelli, M., Sobiczewski, P., & Puławska, J. (2016). Pseudomonas cerasi sp. nov. (non griffin, 1911) isolated from diseased tissue of cherry. Systemic and Applied Microbiology, 39, 370–377.
Kaymak, S., Özdem, A., Karahan, A., Özercan, B., Aksu, P., Aydar, A., et al. (2015). Ülkemizde Zirai Mücadele Girdilerinin Değerlendirilmesi. Ankara, Turkiye. 83. (in Turkish).
Kennelly, M. M., Cazorla, F. M., Vicente, A., Ramos, C., & Sundin, G. W. (2007). Pseudomonas syringae diseases of fruit trees: Progress toward understanding and control. Plant Disease, 91(1), 4–17.
Koh, E., & Henderson, J. P. (2015). Microbial copper-binding Siderephers at the host-pathogen Interface. The Journal of Biological Chemistry, 290(31), 18967–18974.
Kotan, R., & Şahin, F. (2002). First record of bacterial canker caused by Pseudomonas syringae pv. syringae, on apricot trees in Turkey. Plant Pathology, 51(6), 798–798.
Ladomerskyab, E., & Petris, M. J. (2015). Copper tolerance and virulence in bacteria. Metallomics, 7, 957–964.
Lamichhane, J. R., Varvaro, L., Parisi, L., Audergon, J.-M., & Morris, C. E. (2014). Disease and frost damage of woody plants caused by Pseudomonas syringae: Seeing the forest for the trees. Advances in Agronomy, 126, 235–295.
Lamichhane, J. R., Messean, A., & Morris, C. E. (2015). Insights into epidemiology and control of diseases of annual plants caused by the Pseudomonas syringae species complex. Journal of General Plant Pathology, 81, 331–350.
Lamichhane, J. R., Osdaghi, E., Behlau, F., Köhl, J., Jones, J. B., & Aubertot, J. N. (2018). Thirteen decades of antimicrobial copper compounds applied in agriculture. A review. Agronomy for Sustainable Development, 38, 28. https://doi.org/10.1007/s13593-018-0503-9.
Lejon, D. P. H., Nowak, V., Bouko, S., Pascault, N., Mougel, C., Martins, J. M. F., & Ranjard, L. (2007). Fingerprinting and diversity of bacterial copA genes in response to soil types, soil organic status and copper contamination. FEMS Microbiology Ecology, 61(3), 424–437.
Louden, B. C., Haarmann, D., & Lynne, M. A. (2011). Use of blue agar cas assay for siderophore detection. Journal of Microbiology & Biology Education, 12(1), 51–53.
Malik, A., & Jaiswal, R. (2000). Metal resistance in Pseudomonas strains isolated from soil treated with industrial wastewater. World Journal of Microbiology & Biotechnology, 16, 177–182.
Marco, G. M., & Stall, R. E. (1983). Control of bacterial spot of pepper initiated by strains of Xanthomonas campestris pv. vesicatoria that differ in sensitivity to copper. Plant Disease, 67(7), 779–781.
Martins, G., Miot-Sertier, C., Lonvaud-Funel, A., & Masneuf-Pomarede, I. (2016). Grape berry bacterial inhibition by different copper fungicides. BIO web of conferences 7, 01043. 39th world congress of vine and wine. DOI: https://doi.org/10.1051/bioconf/20160701043.
Medhekar, S., & Boparai, K. S. (1981). Fungicidal bis (1-amidino-O-ethylisourea) copper (II) carbamates. Journal of Agricultural and Food Chemistry, 29, 421–422.
Mgbechi-Ezer, U. J., Johnson, B. K., Porter, D. L., & Oraguzie, C. N. (2018). Development of a protocol to phenotype sweet cherry (Prunus avium L.) for resistance to bacterial canker. Crop Protection, 112, 246–251.
Nakajima, M., Goto, M., & Hibi, T. (2002). Similarity between copper resistance genes from Pseudomonas syringae pv. actinidiae and P. syringae pv. tomato. Journal of General Plant Pathology, 68(1), 68–74.
Özaktan, H., Öden, S., Delen, N. (1991), Domates bakteriyel benek hastalığı etmeni (Pseudomonas syringae pv. tomato)‘ne bazı bakırlı preparatların etkililikleri üzerinde araştırmalar, VI.Türkiye Firopatoloji Congress. 7-11 October 1991, İzmir. 291-294. (in Turkish).
Özaktan H., Akkopru A., Bozkurt A., Erdal M., 2008. Information on peach bacterial canker in Aegean Region of Turkey. Proceedings of STF meeting on “Determination of the incidence of the different pathovars of Pseudomonas syringae in stone fruits”. COST Action 873 “Bacterial Diseases of Stone Fruits and Nuts”. 27th–28th March 2008, Skierniewice, Polant.
Parisi, L., Morgaint, B., Blanco-Garcia, J., Guilbaud, C., Chandeysson, C., Bourgeay, J. F., Moronvalle, A., Brun, L., Brachet, M. L., & Morris, C. E. (2019). Bacteria from four phylogroups of the Pseudomonas syringae complex can cause bacterial canker of apricot. Plant Pathology, 68, 1249–1258.
Patel, P. R., Shaikh, S. S., & Sayyed, R. Z. (2018). Modified chrome azurol S method for detection and estimation of siderophores having affinity for metal ions other than iron. Environmental Sustainability, 1(1), 81–87.
Petriccione, M., Zampella, L., Mastrobuoni, F., & Scortichini, M. (2017). Occurrence of copper-resistant Pseudomonas syringae pv. syringae strains isolated from rain and kiwifruit orchards also resistance in Pseudomonas syringae pv. Syringae. European Journal of Plant Pathology, 149(4), 953–968.
Renick, L. J., Cogal, A. G., & Sundin, G. W. (2008). Phenotypic and genetic analysis of epiphytic Pseudomonas syringae populations from sweet cherry in Michigan. Plant Disease, 92, 372–378.
Rensing, C., & Grass, G. (2003). Escherichia coli mechanisms of copper homeostasis in a changing environment. FEMS Microbiology Reviews, 27, 197–213.
Scheck, H. J., & Pscheidt, J. W. (1998). Effect of copper bactericides on copper-resistant and -sensitive strains of Pseudomonas syringae pv. syringae. Plant Disease, 82(4), 397–406.
Schwyn, B., & Neiland, J. B. (1987). Universal chemical assay for the detection and determination of siderophores. Analytical Biochemistry, 160(1), 47–56.
Scortichini, M. (2010). Epidemiology and predisposing factors of some major bacterial diseases of stone and nut fruit trees species. Journal of Plant Pathology, 92(1), 73–78.
Smalla, K., Haines, A. S., Jones, K., Krögerrecklenfort, E., Heuer, H., Schloter, M., & Thomas, C. M. (2006). Increased abundance of IncP-1β plasmids and mercury resistance genes in mercury-polluted river sediments: First discovery of IncP-1β plasmids with a complex mer transposon as the sole accessory element. Applied and Environmental Microbiology, 72, 7253–7259.
Spotts, R. A., Wallis, K. M., Serdani, M., & Azarenko, A. N. (2010a). Bacterial canker of sweet cherry in Oregon—Infection of horticultural and natural wounds, and resistance of cultivar and rootstock combinations. Plant Disease, 94, 345–350.
Spotts, R.A., Olsen, J.L., Long, L.E., Pscheidt, J.W. (2010b). Bacterial canker of sweet cherry in Oregon: Disease symptoms, cycle, and management. Oreg. State Univ. Ext. Bull 1–4.
Sundin, G. W., Jones, A. L., & Fulbright, D. W. (1989). Copper resistance in Pseudomonas syringae pv. syringae from cherry orchards and its associated transfer in-vitro and in planta with a plasmid. Phytopathology, 79(8), 861–865.
Sundin, G. W., Demezas, D. H., & Bender, C. L. (1994). Genetic and plasmid diversity within natural populations of Pseudomonas syringae with various exposures to copper and streptomycin bactericides. Applied and Environmental Microbiology, 60(12), 4421–4431.
Teitzel, G. M., Geddie, A., De Long, S. K., Kirisits, M. J., Whiteley, M., & Parsek, M. R. (2006). Survival and growth in the presence of elevated copper: Transcriptional profiling of copper-stressed Pseudomonas aeruginosa. Journal of Bacteriology, 188(20), 7242–7256.
Vicente, J. G., Roberts, S. J., Russell, K., & Alves, J. P. (2004). Identification and discrimination of Pseudomonas syringae isolates from wild cherry in England. European Journal of Plant Pathology, 110, 337–351.
Visca, P., Colotti, G., Serino, L., Verzili, D., Orsi, N., & Chiancone, E. (1992). Metal regulation of siderophore synthesis in Pseudomonas aeruginosa and functional effects of siderophore-metal complexes. Applied and Environmental Microbiology, 58, 2886–2893.
Wenneker, M., Janse, J. D., & Bruine, J. A. (2012). Bacterial canker of plum trees, caused by Pseudomonas syringae pathovars, as a serious threat for plum production in the Netherlands. Journal of Plant Pathology, 76, 575–578.
Yoon, S., Dispirito, A.A., Kraemer, S.M., & Semrau, J. D. (2011). A simple assay for screening microorganisms for chalkophore production, chap. 16. Methods in Enzymology, USA. 495.
Acknowledgements
This work was supported by the Scientific Research Project Units of Van Yuzuncu Yil University (grant numbers: FAP-2018-7627). We would like to thank Prof. Dr. Hatice ÖZAKTAN for isolates of Pseudomonas syringae pathovars isolates supply.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Husseini, A., Akköprü, A. The possible mechanisms of copper resistance in the pathogen Pseudomonas syringae pathovars in stone fruit trees. Phytoparasitica 48, 705–718 (2020). https://doi.org/10.1007/s12600-020-00828-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12600-020-00828-1