Abstract
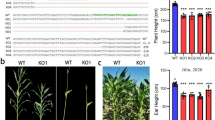
Glutathione S-transferases (GSTs) are multifunctional proteins that play important roles in cellular detoxification as well as in plant growth and development. Here, we cloned a new rice tau class GST gene, OsGSTU6, and evaluated its roles in regulating cadmium (Cd) stress tolerance of rice. OsGSTU6 is located both in the cytosol and nucleus of cells. The transcripts of OsGSTU6 were detected in the whole life cycle of rice with higher levels in the leaf blade and sheath at heading stage. The transcripts of OsGSTU6 were significantly stimulated by a number of environmental factors including several phytohormones, abiotic stresses, and heavy metals, suggesting its stress-responsive characteristics. Moreover, the overexpression (OE) of OsGSTU6 in rice reduced the accumulation of Cd in leaf and enhanced the tolerance of the plant to Cd stress, while knockdown (RNAi) of OsGSTU6 triggered Cd accumulation in rice leaves and decreased stress tolerance. Meanwhile, the expression levels of several candidate genes responsible for scavenging reactive oxygen species (ROS) were downregulated in OE of OsGSTU6 lines. In addition, the content of superoxide anions (O2−) and glutathione showed reduction in OE plants, but was augmented in RNAi plants under both normal growth and Cd stress, compared with wild type. Furthermore, both the yeast two-hybrid assay and firefly luciferase complementation imaging (LCI) assay demonstrated that OsGSTU6 might function with dimers in plant cells. All these results suggest that OsGSTU6 may play an important role in Cd stress tolerance of rice by involving in the intracellular ROS homeostasis.







Similar content being viewed by others
References
Adamis PD, Gomes DS, Pinto MLC, Panek AD, Eleutherio EC (2004) The role of glutathione transferases in cadmium stress. Toxicol Lett. 154:81–88
Agrawal GK, Jwa NS, Eakwal R (2002) A pathogen-induced novel rice (Oryza sativa L.) gene encodes a putative protein homologous to type II glutathione Stransferases. Plant Sci 12:1939–1950
Anderson JV, Davis DG (2004) Abiotic stress alters transcript profiles and activity of glutathione S-transferase, glutathione peroxidase, and glutathione reductase in Euphorbia esula. Physiol Plant 120:421–433
Apel K, Hirt H (2004) Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu Rev Plant Biol 55:373–399
Apse MP, Blumwald E (2002) Engineering salt tolerance in plants. Curr Opin Biotech 3:146–150
Asada K (1999) The water-water cycle in chloroplasts: scavenging of activeoxygen and dissipation of excess photons. Annu Rev Plant Physiol Plant Mol Biol 50:601–639
Bai XJ, Liu LJ, Zhang CH, Ge Y, Cheng WD (2011) Effect of H2O2 pretreatment on Cd tolerance of different rice cultivars. Rice Sci 18:29–35
Berhane K, Widersten M, EngströmKozarich MAJW, B, Mannervik (1994) Detoxication of base propenals and other alpha, beta-unsaturated aldehyde products of radical reactions and lipid peroxidation by human glutathione transferases. Proc Natl Acad Sci 91:1480–1484
Bianchi MW, Roux C, Vartanian N (2002) Drought regulation of GST8, encodes the Arabidopsis homologue of ParC/Nt107 glutathione transferase/ peroxidase. Physiol Plant 116:96–105
Cecconi I, Scaloni A, Rastelli G, Moroni M, Vilardo PG, Costantino L, Cappiello M, Garland D, Carper D, Petrash JM (2002) Oxidative modification of aldose reductase induced by copper ion. Definition of the metal-protein interaction mechanism. J Biol Chem 277(44):42017–42027
Chen IC, Huang IC, Liu MJ, Wang ZG, Chung SS, Hsieh HL (2007) Glutathione Stransferase interacting with far-red insensitive 219 is involved in phytochromeAmediated signaling in Arabidopsis. Plant Physiol 143:1189–1202
Chen YF, Li LQ, Xu Q, Kong YH, Wang H, Wu WH (2009) The WRKY6 transcription factor modulates PHOSPHATE1 expression in response to low Pi stress in Arabidopsis. Plant Cell 21:3554–3566
Csiszár J, Horváth E, Váry Z, Gallé Á, Bela K, Brunner S, Tari I (2014) Glutathione transferase supergene family in tomato: Salt stress-regulated expression of representative genes from distinct GST classes in plants primed with salicylic acid. Plant Physiol Biochem 78:15–26. https://doi.org/10.1016/j.plaphy.2014.02.010
Das K, Roychoudhury A (2014) Reactive oxygen species (ROS) and response of antioxidants as ROS-scavengers during environmental stress in plants. Front Environ Sci 2:53
Ding N, Wang AM, Zhang XJ, Wu YX, Wang RY, Cui HH, Huang RL, Luo YH (2017) Identification and analysis of glutathione S-transferase gene family in sweet potato reveal divergent GST-mediated networks in aboveground and underground tissues in response to abiotic stresses. BMC Plant Biol 17:225
Dixit P, Mukherjee PK, Ramachandran V, Eapen S (2011) Glutathione transferase from Trichoderma virens enhances cadmium tolerance without enhancing its accumulation in transgenic Nicotiana tabacum. PLoS ONE 6(1):e16360
Dixon DP, Adrian L, Robert E (2002) Plant glutathione transferases. Genome Biol 401(3):169–186
Dixon DP, Cole DJ, Edwards R (1999) Dimerisation of maize glutathione transferases in recombinant bacteria. Plant Mol Biol 40:997–1008
Dixon DP, Skipsey M, Edwards R (2010) Roles for glutathione transferases in plant secondary metabolism. Phytochemistry 71:338–350
Dong Y, Li C, Zhang Y, He Q, Daud MK, Chen J (2016) Glutathione S-transferase gene family in Gossypiumraimondii and G. arboreum: comparative genomic study and their expression under salt stress. Front. Plant Sci. 7:139
Duan ZQ, Bai L, Zhao ZG, Zhang GP, Cheng FM, Jiang LX, Chen KM (2009) Drought-stimulated activity of plasma membrane nicotinamide adenine dinucleotide phosphate oxidase and its catalytic properties in rice. J Integr Plant Biol 51:1104–1115
Edwards R, Dixon DP (2005) Plant glutathione transferases. Methods Enzymol. 401:169–186
Edwards R, Dixon DP, Walbot V (2000) Plant glutathione S-transferases: enzymes with multiple functions in sickness and in health. Trends Plant Sci 5(5):193–198
Ezaki B, Suzuki M, Motoda H, Kawamura M, Nakashima S, Matsumoto H (2004) Mechanism of gene expression of Arabidopsis glutathione S-transferase, AtGST1 and AtGST11 in response to aluminum stress. Plant Physiol 134:1672–1682
Ezaki B, Yamamoto Y, Matsumoto H (1995) Cloning and sequencing of the cDNAs induced by aluminium treatment and Pi starvation in tobacco cultured cells. Physiol Plant 93:11–18
Gill SS, Tuteja N (2010) Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Bioch. 48:909–930
Gronwald JW, Plaisance KL (1998) Isolation and characterization of GlutathioneS-transferaseisozymes from sorghum. Plant Physiol 117:877–892
Halliwell B (1987) Oxidative damage, lipid peroxidation and antioxidantprotection in chloroplasts. Chem Phys Lipids 44:327–340
Halliwell B, Gutteridge JMC (1993) Free radicals in biology and medicine. Clarendon Press, Oxford
Hayes JD, Flanagan JU, Jowsey IR (2005) Glutathione transferases. Annu Rev Pharmacol Toxicol 45:51–88
He G, Guan CN, Chen QX, Gou XJ, Liu W, Zeng QY (2016) Genome-wide analysis of the glutathione S-transferase gene family in Capsella rubella: identification, expression, and biochemical functions. Front Plant Sci 7:1325
Hegedüs A, Erdei S, Horváth G (2001) Comparative studies of H2O2 detoxifying enzymes in green and greening barley seedling under Cd stress. Plant Sci. 160:1085–1093
Hoagland DR, Arnon DI (1950) The water-culture method for growing plants without soil. Circ Calif Agric Exp Stn 347:32
Hu Y, Ge Y, Zhang C, Ju T, Cheng W (2009) Cadmium toxicity and translocation in rice seedlings are reduced by hydrogen peroxide pretreatment. Plant Growth Regul 59:51
Izhar M, Jing XQ, Abdullah S, Muhammad A, Shi Y, Gan PF, Li WQ, Liu WT, Chen KM (2018) Comparative in silico analysis of ferric reduction oxidase (FRO) genes expression patterns in response to abiotic stresses, metal and hormone applications. Molecules 23:1163. https://doi.org/10.3390/molecules23051163
Jiang M, Zhang J (2003) Cross-talk between calcium and reactive oxygen species originated from NADPH oxidase in abscisic acid-induced antioxidant defence in leaves of maize seedlings. Plant Cell Environ 26(6):929–939
Jain M, Ghanashyam C, Bhattacharjee A (2010) Comprehensive expression analysis suggests overlapping and specific roles of glutathione S-transferases during development and stress responses in rice. BMC Genomics 11:73
Jefferson RA, Kavanagh TA, Bevan MW (1987) GUS fusions: β-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J 6:3901–3907
Jha B, Sharma A, Mishra A (2011) Expression of SbGSTU (tau class glutathione S-transferase) gene isolated from Salicorniabrachiata in tobacco for salt tolerance. Mol Biol Rep 38:4823–4832
Kawasaki T, Koita H, Nakatsubo T, Hasegawa K, Wakabayashi K, Takahashi H, Umemura K, Umezawa T, Shimamoto K (2006) Cinnamoyl-CoA reductase, a key enzyme in lignin biosynthesis, is an effector of small GTPase Rac in defense signaling in rice. Proc Natl Acad Sci USA 103:230–235
Kimiti KG, Atanassova N, Vardanyan A, Clatot N, Al-Sabarna K, Kanellopoulos PN, Makris AM, Kampranis SC (2004) Differential roles of tau class glutathione S-transferases in oxidative stress. J Biol Chem 279(23):24540–24551
Klinedinst S, Pascuzzi P, Redman J, Desai M, Arias J (2000) A xenobiotic-stress-activated transcription factor and its cognate target genes are preferentially expressed in root tip meristems. Plant Mol Biol 42:679–688
Kozhevnikova AD, Seregin IV, Bystrova EI, Belyaeva AI, Kataeva MN, Ivanov VB (2009) The effects of lead, nickel, and strontium nitrates on cell division and elongation in maize roots. Russ J Plant Physiol 56(2):242–250
Kunieda T, Fujiwara T, Amano T, Shioi Y (2005) Molecular cloning and characterization of a senescence-induced tau-class glutathione S-transferase from barley leaves. Plant cell physiol 46:1540–1548
Levine A, Tenhaken R, Dixon R, Lamb C (1994) H2O2 from the oxidative burst orchestrates the plant hypersensitive disease resistance response. Cell 79:583–593
Li ZK, Chen B, Li XX, Wang JP, Zhang Y, Wang XF, Yan YY, Ke HF, Yang J, Wu JH, Wang GN, Zhang GY, Wu LQ, Wang XY, Ma ZY (2018) A newly-identified cluster of glutathione S-transferase genes provides Verticillium wilt resistance in cotton. Plant J. https://doi.org/10.1111/tpj.14206
Licciardello C, D’Agostino N, Traini A, Recupero GR, R Frusciante L, Chiusano ML (2014) Characterization of the glutathione S-transferase gene family through ESTs and expression analyses within common and pigmented cultivars of Citrus sinensis (L.) Osbeck. BMC Plant Biol 14(1):39
Loyall L, Uchida K, Braun S, Furuya M, Frohnmeyer H (2000) Glutathione and a UV light-induced glutathione S-transferase are involved in signaling to chalcone synthase in cell cultures. Plant Cell 12:1939–1950
Lu X, Wang C, Liu BZ (2015) The role of Cu/Zn-SOD and Mn-SOD in the immune response to oxidative stress and pathogen challenge in the calm Meretrixmeretrix. Fish Shellfish Immun 42:58–65
Mannervik B (2010) Glutathione transferase. Annu Rev Pharmacol 8(1):0131
Marrs KA, Walbot V (1997) Expression and RNA splicing of the maize glutathione S-transferase Bronze2 gene is regulated by cadmium and other stresses. Plant Physiol 113:93–102
Mauch F, Dudler R (1993) Differential induction of distinct glutathione-S-transferases of wheat by xenobiotics and by pathogen attack. Plant Physiol 102:1193–1201
McGonigle B, Keeler SJ, Lau SM, Koeppe MK, O’Keefe DP (2000) A genomics approach to the comprehensive analysis of the glutathione S-transferase gene family in soybean and maize. Plant Physiol. 124:1105–1120
Miller G, Suzuki N, Ciftci-Yilmaz S, Mittler R (2010) Reactive oxygen specieshomeostasis and signalling during drought and salinity stresses. Plant Cell Environ 33:433–685
Mittler R (2002) Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci 7:405–410
Mohsenzadeh S, Esmaeili M, Moosavi F, Shahrtash M, Saffari B, Mohabatkar H (2011) Plant glutathione S-transferase classification, structure and evolution. Afr J Biotech 10(42):8160–8165
Moons A (2003) Osgstu3 and Osgstu4, encoding tau class glutathione Stransferases, are heavy metal- and hypoxic stress-induced and differentially salt stress-responsive in rice roots. FEBS Lett 553:427–432
Mordacq JC, Roberta E (2008) The yeast two-hybrid assay. Scientist 19(16):32
Nocito FF, Lancilli C, Dendena B, Lucchini G, Sacchi GA (2011) Cadmium retention in rice roots is influenced by cadmium availability, chelation and translocation. Plant Cell Environ 34:994–1008
Qi YC, Liu WQ, Qiu LY, Zhang SM, Ma L, Zhang H (2010) Overexpression of glutathione S-transferase gene increases salt tolerance of Arabidopsis. Russ J Plant Physl 57:233–240
Rahman I, Kode A, Biswas SK (2006) Assay for quantitative determination of glutathione and glutathione disulfide levels using enzymatic recycling method. Nat Protoc 1:3159–3165
Rezaei MK, Shobbar ZS, Shahbazi M, Abedini R, Zare S (2013) Glutathione S-transferase (GST) family in barley: identification of members, enzyme activity, and gene expression pattern. J Plant Physiol 170(14):1277–1284
Roxas VP, Lodhi SA, Garrett DK, Mahan JR, Allen RD (2000) Stress tolerancein transgenic tobacco seedlings that overexpress glutathione S-transferase/glutathione peroxidase. Plant Cell Physiol 41:1229–1234
Roxas VP, Smith RKJ, Allen ER, Allen RD (1997) Overexpression of glutathione S-transferase/glutathione peroxidase enhances the growth of transgenic tobacco seedlings during stress. Nat Biotechnol 15:988–991
Sanitádi TL, Lambardi M, Pecchioni N, Pazzagli L, Durante M (1999) Effects of cadmium stress on hairy roots of Daucuscarota. J Plant Physiol 154:385–391
Sappl PG, Carroll AJ, Clifton R, Lister R, Whelan J, Harvey MA (2009) The Arabidopsis glutathione transferase gene family displays complex stress regulation and co-silencing multiple genes results in altered metabolic sensitivity to oxidative stress. Plant J 58:53–68
Shalmani A, Jing X-Q, Shi Y, Muhammad I, Zhou M-R, Wei X-Y, Chen Q-Q, Li W-Q, Liu W-T, Chen K-M (2019) Characterization of B-BOX gene family andtheir expression profiles under hormonal, abiotic and metal stresses in Poaceae plants. BMC Genomics 20:27
Sheehan D, Meade G, Foley VM, Dowd CA (2001) Structure, function and evolution of glutathione transferases: implications for classification of non-mammalian members of an ancient enzyme superfamily. Biochem J 360:1–16
Shi HY, Li ZH, Zhang YX, Chen L, Xiang DY, Zhang YF (2014) Two pear glutathione S-transferases genes are regulated during fruit development and involved in response to salicylic acid, auxin, and glucose signaling. PLoS ONE 9:e89926
Shigeoka S, Ishikawa T, Tamoi M, Miyagawa Y, Takeda T, Yabuta Y, Yoshimura k, (2002) Regulation and function of ascorbate peroxidase isoenzymes. J Exp Bot 53:1305–1319
Soranzo N, Gorla MS, Mizzi L, De Toma G, Frova C (2004) Organisation and structural evolution of the rice glutathione S-transferase gene family. Mol Genet Genomics 271:511–521
Sun K, Zheng Y, Zhu Z (2017) Luciferase complementation imaging assay in Nicotiana benthamiana leaves for transiently determining protein-protein interaction dynamics. J Vis Exp 129:e56641
Thom R, Cummins I, Dixon DP, Edwards R, Cole DJ, Lapthorn AJ (2002) Structure of a Tau class glutathione S-transferase from wheat active in herbicide detoxification. Biochemistry 41:7008–7020
Toki S, Hara N, Ono K, Onodera H, Tagiri A, Oka S, Tanaka H (2006) Early infection of scutellum tissue with Agrobacterium allows high-speed transformation of rice. Plant J. 47:969–976
Uraguchi S, Mori S, Kuramata M, Kawasaki A, Arao T, Ishikawa S (2009) Root-to-shoot Cd translocation via the xylem is the major process determining shoot and grain cadmium accumulation in rice. J Exp Bot 60:2677–2688
Vollenweider S, Weber H, Stolz S, Chételat A, Farmer EE (2000) Fatty acid ketodienes and fatty acid ketotrienes: michael addition acceptors that accumulate in wounded and diseased Arabidopsis leaves. Plant J 24:467–476
Wagner U, Edwards R, Dixon DP, Mauch F (2002) Probing the diversity of the Arabidopsis glutathione S-transferase gene family. Plant mol biol 49:515–532
Wang X, Zhang MM, Wang YJ, Gao YT, Li R, Wang GF, Li WQ, Liu WT (2016) The plasma membrane NADPH oxidase OsRbohA plays a crucial role in developmental regulation and drought-stress response in rice. Physiol Plant 156(4):421–443
Wong CKE, Cobbett CS (2009) HMA P-type ATPases are the major mechanism for root-to-shoot Cd translocation in Arabidopsis thaliana. New Phytol 181:71–78
Xu F, Lagudah ES, Moose SP, Riechers DE (2002) Tandemly duplicated Safener-induced glutathione S-transferase genes from Triticumtauschii contribute to genome -and organ specific expression in hexaploid wheat. Plant Physiol 130:362–373
Xu J, Tian YS, Xing XJ, Peng RH, Zhu B, Gao JJ, Yao H (2016) Over-expression of AtGSTU19 provides tolerance to salt, drought and methyl viologen stressesin Arabidopsis. Physiol Plant 156:164–175
Yamaguchi K, Imai K, Akamatsu A, Mihashi M, Hayashi N, ShimamotoK., Kawasaki T., (2012) SWAP70 functions as a Rac/Rop guanine nucleotide-exchange factor in rice. Plant J 70:389–397
Yang Q, Liu YJ, Zeng QY (2014) Biochemical functions of the glutathione transferase supergene family of Larixkaempferi. Plant Physiol Bioch 77:99–107
Zhou J, Goldsbrough PB (1993) An Arabidopsis gene with homology to glutathione S-transferase is regulated by ethylene. Plant Mol Biol 22:517–523
Acknowledgements
This work was supported by the Shaanxi Provincial Key Project of Research and Development Plan, China (2019SF-249) and the National Natural Science Foundation of China (Grant Nos. 31770204 and 31270299). The funders had no role in the designing and performing the experiments.
Author information
Authors and Affiliations
Contributions
X-QJ and M-RZ have contributed equally to this work. K-MC, X-QJ, and M-RZ conceived and designed the research. X-QJ and M-RZ performed the experiments, analyzed the data, and wrote the article. HM cloned the sequence and constructed the transgenic lines. P-TS and AS cultivated rice plants and extracted RNA. X-MN and LZ constructed vectors. K-MC and W-TL improved and commented on the article. K-MC supported the research. All authors read the article and approved the final version.
Corresponding authors
Ethics declarations
Conflict interest
The authors declare no conflict interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
344_2020_10148_MOESM1_ESM.tif
Survival rates statistics of OsGSTU6-transgenic plants treated with 1 mM Cd for 14 days. Data are means ±SD from three independent biological replicates (We planted seeds on April 2, August 20 and November 5, 2018, respectively, and then carried out the experiments and obtained results, as shown in A, B and C). Supplementary file1 (TIF 10743 kb)
Rights and permissions
About this article
Cite this article
Jing, XQ., Zhou, MR., Nie, XM. et al. OsGSTU6 Contributes to Cadmium Stress Tolerance in Rice by Involving in Intracellular ROS Homeostasis. J Plant Growth Regul 40, 945–961 (2021). https://doi.org/10.1007/s00344-020-10148-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00344-020-10148-7