Abstract
The AMT1 family comprises major ammonium transporters in rice roots. In this study, we utilized AMT1 RNAi mutants (amt1) to explore how AMT1 affects NH4+- and NO3–-mediated morphological development and NH4+-responsive gene expression in roots. In the presence of NH4+, amt1 showed inhibition of NO3–- dependent lateral root development. The inhibitory action of NH4+ on lateral root growth was independent of the NO3– concentrations supplied to amt1 roots. The results of split root assays indicated that NH4+ exerts systemic action in inhibiting NO3–-dependent lateral root development in amt1. Further study with NAA and NOA, a potent auxin flux inhibitor, suggested that perturbation of membrane dynamics might not be the primary cause of the inhibitory action of NH4+ on NO3–-mediated lateral root growth in amt1 mutants. RNA-seq analysis of NH4+-responsive genes showed that approximately half of DEGs observed in wild-type roots were not detected in the DEGs of amt1 roots. Gene ontology enrichment analysis suggested that the expression of specific functional gene groups were affected by amt1 during the early response to NH4+. Auxin-responsive gene expression and root gravity responses were altered in amt1. This study demonstrated that AMT1 affects the interactions not only between ammonium and nitrate in lateral root growth but also between auxin and NH4+ in rice roots.


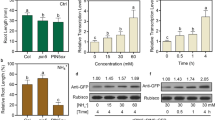
source for 14 d. Fresh nutrient solutions were provided every 2 days. a, b, c, d show quantification of seminal root length, crown root numbers, lateral root density, and lateral root length, respectively. Crown roots longer than 0.5 cm were counted separately from those shorter than 0.5 cm. e Crown roots grown in ¼ NS containing NH4+. b Each bar consists of a number of crown roots longer than 0.5 cm (lower part) and those shorter than 0.5 cm (upper part). Red arrows in (e) indicate crown roots shorter than 0.5 cm. Scale bar = 1 cm. Data of (a, b, c, d) are means ± SE (n > 10 plants per line). Different letters indicate significant differences between samples (P < 0.05)







Similar content being viewed by others
References
Abiko T, Obara M, Ushioda A, Hayakawa T, Hodges M, Yamaya T (2005) Localization of NAD-isocitrate dehydrogenase and glutamate dehydrogenase in rice roots: candidates for providing carbon skeletons to NADH-glutamate synthase. Plant Cell Physiol 46:1724–1734
Araya T, Kubo T, von Wirén N, Takahashi H (2016) Statistical modeling of nitrogen-dependent modulation of root system architecture in Arabidopsis thaliana. J Integr Plant Biol 58:254–265
Bao AZ, Liang Z, Zhao CH (2015) Overexpressing of OsAMT1-3, a high affinity ammonium transporter gene, modifies rice growth and carbon-nitrogen metabolic status. Int J Mol Sci 16(5):9037–9063
Barth C, Gouzd ZA, Steele HP, Imperio RM (2010) A mutation in GDP-mannose pyrophosphorylase causes conditional hypersensitivity to ammonium, resulting in Arabidopsis root growth inhibition, altered ammonium metabolism, and hormone homeostasis. J Exp Bot 61(2):379–394
Bouguyon E, Perrine-Walker F, Pervent M, Rochette J, Cuesta C, Benkova E, Nacry P (2016) Nitrate controls root development through posttranscriptional regulation of the NRT1.1/NPF6.3 transporter/sensor. Plant Physiol 172(2):1237–1248. https://doi.org/10.1104/pp.16.01047
Brady SM, Burow M, Busch W, Carlborg Ö, Denby KJ, Glazebrook J, Hamilton ES, Harmer SL, Haswell ES, Maloof JN (2015) Reassess the t test: interact with all your data via ANOVA. Plant Cell 27:2088–2094
Britto DT, Kronzucker HJ (2002) NH4+ toxicity in higher plants: a critical review. J Plant Physiol 159:567–584
Britto DT, Siddiqi MY, Glass AD, Kronzucker HJ (2001) Futile transmembrane NH4+ cycling: a cellular hypothesis to explain ammonium toxicity in plants. Proc Natl Acad Sci USA 98(7):4255–4258
Cao Y, Glass AD, Crawford NM (1993) Ammonium inhibition of Arabidopsis root growth can be reversed by potassium and by auxin resistance mutations aux1, axr1, and axr2. Plant Physiol 102:983–989
Cerezo M, Tillard P, Gojon A, Primo-Millo E, Garcia-Agustin P (2001) Characterization and regulation of ammonium transport systems in Citrus plants. Planta 214:97–105
Chaudhuri B, Hormann F, Lalonde S, Brady SM, Orlando DA, Benfey P, Frommer WB (2008) Protonophore- and pH-insensitive glucose and sucrose accumulation detected by FRET nanosensors in Arabidopsis root tips. Plant J 56:948–962
Chen RF, Shen RF, Gu P, Dong XY, Du CW, Ma JF (2006) Response of rice (Oryza sativa) with root surface iron plaque under aluminium stress. Ann Bot 98(2):389–395
Chin HC, Choe MS, Lee SH, Park SH, Park SH, Koo JC, Kim NY, Lee JJ, Oh BG, Yi GH, Kim SC, Choi HC, Cho MJ, Han CD (1999) Molecular analysis of rice plants harboring an Ac/Ds transposable element-mediated gene trapping system. Plant J 19(5):615–623
Fernández-Crespo E, Scalschi L, Llorens E, García-Agustín P, Camañes G (2015) NH4+ protects tomato plants against Pseudomonas syringae by activation of systemic acquired acclimation. J Exp Bot 66:6777–6790
Gaur VS, Singh US, Gupta AK, Kumar A (2012) Understanding the differential nitrogen sensing mechanism in rice genotypes through expression analysis of high and low affinity ammonium transporter genes. Mol Biol Rep 39:2233–2241. https://doi.org/10.1007/s11033-011-0972-2
Hirano T, Satoh Y, Ohki A, Takada R, Arai T, Michiyama H (2008) Inhibition of ammonium assimilation restores elongation of seminal rice roots repressed by high levels of exogenous ammonium. Physiol Plant 134:183–190
Husted S, Schjoerring JK (1995) Apoplastic pH and ammonium concentration in leaves of Brassica napus L. Plant Physiol 109(4):1453–1460
Imhoff V, Muller P, Guern J, Delbarre A (2000) Inhibitors of the carrier-mediated influx of auxin in suspension-cultured tobacco cells. Planta 210:580–588
Kim EJ, Kim YJ, Hong WJ, Jeon JS, Jung KH (2019) Genome-wide analysis of root hair preferred RBOH genes suggests that three RBOH genes are associated with auxin-mediated root hair development in rice. J Plant Biol 62:229–238
Kronzucker HJ, Siddiqi MY, Glass ADM, Kirk GJD (1999a) Nitrate ammonium synergism in rice: a subcellular analysis. Plant Physiol 119:1041–1046
Kronzucker HJ, Glass AD, Yaeesh Siddiqi M (1999b) Inhibition of nitrate uptake by ammonium in barley. Analysis component fluxes. Plant Physiol 120(1):283–292. https://doi.org/10.1104/pp.120.1.283
Lankova M, Smith R, Pesek B, Kubes M, Zazimalova E, Petrasek J (2010) Auxin influx inhibitors 1-NOA, 2-NOA and CHPAA interfere with membrane dynamics in tobacco cells. J Exp Bot 61:3589–3598. https://doi.org/10.1093/jxb/erq172
Li Q, Li BH, Kronzucker HJ, Shi WM (2010) Root growth inhibition by NH4+ in Arabidopsis is mediated by the root tip and is linked to NH4+ efflux and GMPase activity. Plant Cell Environ 33:1529–1542
Li B, Li Q, Su Y, Chen H, Xiong L, Mi G, Kronzucker HJ, Shi W (2011) Shoot-supplied ammonium targets the root auxin influx carrier AUX1 and inhibits lateral root emergence in Arabidopsis. Plant Cell Environ 34:933–946
Li B, Li Q, Xiong L, Kronzucker HJ, Kramer U, Shi W (2012) Arabidopsis plastid AMOS1/EGY1 integrates abscisic acid signaling to regulate global gene expression response to ammonium stress. Plant Physiol 160:2040–2051
Li G, Li B, Dong G, Feng X, Kronzucker HJ, Shi W (2013) Ammonium-induced shoot ethylene production is associated with the inhibition of lateral root formation in Arabidopsis. J Exp Bot 64:1413–1425
Lima JE, Kojima S, Takahashi H, von Wirén N (2010) Ammonium triggers lateral root branching in Arabidopsis in an AMMONIUM TRANSPORTER1;3-dependent manner. Plant Cell 22:3621–3633
Little DY, Rao H, Oliva S, Daniel-Vedele F, Krapp A, Malamy JE (2005) The putative high-affinity nitrate transporter NRT2.1 represses lateral root initiation in response to nutritional cues. Proc Natl Acad Sci USA 102:13693–13698
Liu Y, von Wiren N (2017) Ammonium as a signal for physiological and morphological responses in plants. J Exp Bot 68(10):2581–2592
Liu L, Mei Q, Yu Z, Sun T, Zhang Z, Chen M (2013) An integrative bioinformatics framework for genome-scale multiple level network reconstruction of rice. J Integr Bioinf 10(2):223
Loque D, von Wiren N (2004) Regulatory levels for the transport of ammonium in plant roots. J Exp Bot 55(401):1293–1305
Moon S, Chandran AKN, Kim YJ, Gho Y, Hong WJ, An G, Lee C, Jung KH (2019) Rice RHC encoding a putative cellulase is essential for normal root hair elongation. J Plant Biol 62:82–91
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Plant Physiol 15:473–497
Oliveira IC, Brears T, Knight TJ, Clark A, Coruzzi GM (2002) Overexpression of cytosolic glutamine synthetase. relation to nitrogen, light, and photorespiration. Plant Physiol 129(3):1170–1180.
Patterson K, Cakmak T, Cooper A, Lager I, Rasmusson AG, Escobar MA (2010) Distinct signalling pathways and transcriptome response signatures differentiate ammonium- and nitrate-supplied plants. Plant Cell Environ 33:1486–1501
Ranathunge KA, El-Kereamy S, Gidda Y, Bi M, Rothstein SJ (2014) AMT1;1 transgenic rice plants with enhanced NH4+ permeability show superior growth and higher yield under optimal and suboptimal NH4+ conditions. J Exp Bot 65(4):965–979
Remans T, Nacry P, Pervent M, Filleur S, Diatloff E, Mounier E et al (2006) The Arabidopsis NRT1.1 transporter participates in the signaling pathway triggering root colonization of nitrate-rich patches. Proc Natl Acad Sci USA 103:19206–19211
Shimizu H, Tanabata T, Xie X, Inagaki N, Takano M, Shinomura T, Yamamoto KT (2009) Phytochrome-mediated growth inhibition of seminal roots in rice seedlings. Physiol Plantarum 137:289–297
Sonoda Y, Ikeda A, Saiki S, von Wiren N, Yamaya T, Yamaguchi J (2003) Distinct expression and function of three ammonium transporter genes (OsAMT1;1–1;3) in rice. Plant Cell Physiol 44(7):726–734
Suenaga A, Moriya K, Sonoda IA, Von Wiren N, Hayakawa T, Yamaguchi J, Yamaya T (2003) Constitutive expression of a novel-type ammonium transporter OsAMT2 in rice plants. Plant Cell Physiol 44(2):206–211
Sun CH, Yu JQ, Hu DG (2017) A crucial signal during lateral roots development. Front Plant Sci 8:448
Wang MY, Glass ADM, Shaff JE, Kochian LV (1994) Ammonium uptake by rice roots (III. Electrophysiology). Plant Physiol 104(3):899–906
Xuan YH, Priatama RA, Huang J, Je BI, Liu JM, Park SJ, Piao HL, Son DY, Lee JJ, Park SH, Jung KH, Kim TH, Han CD (2013) Indeterminate domain 10 regulates ammonium-mediated gene expression in rice roots. New Phytol 197:791–804
Xuan YH, Duan FY, Je BI, Kim CM, Li TY, Liu JM, Park SJ, Cho JH, Kim TH, von Wiren N, Han CD (2017) Related to ABI3/VP1-Like 1 (RAVL1) regulates brassinosteroid-mediated activation of AMT1;2 in rice (Oryza sativa). J Exp Bot 68(3):727–737
Xuan YH, Kumar V, Zhu XF et al (2018) IDD10 is involved in the interaction between NH4+ and auxin signaling in rice roots. J Plant Biol 61:72–79
Xuan YH, Kumar V, Han X, Kim SH, JeongJH KCM, Gao Y, Han CD (2019) CBL-INTERACTING PROTEIN KINASE 9 regulates ammonium-dependent root growth downstream of IDD10 in rice (Oryza sativa). Ann Bot 124:947–960
Zou N, Li B, Dong G, Kronzucker HJ, Shi W (2012) Ammonium induced loss of root gravitropism is related to auxin distribution and TRH1 function, and is uncoupled from the inhibition of root elongation in Arabidopsis. J Exp Bot 63:3777–3788
Zou N, Li B, Chen H, Su Y, Kronzucker HJ, Xiong L, Baluska F, Shi W (2013) GSA-1/ARG1 protects root gravitropism in Arabidopsis under ammonium stress. New Phytol 200:97
Acknowledgements
This research was supported by grants from the Next-Generation BioGreen 21 Program (PJ01326601), the Rural Development Administration, Republic of Korea, and from Support Plan for Innovative Talents in Colleges and Universities of Liaoning Province [LR2017037].
Author information
Authors and Affiliations
Contributions
CDH and YHX experimental design, data analysis and interpretation, manuscript editing. VK and SHK data generation and analysis, image analysis, data presentation, manuscript writing. RAP, JHJ, MRA, BAS material generation and analysis, DNA extraction, qPCR analysis. KHJ bioinformatics analysis of RNA-seq data. CMK, BIJ, and SJP data analysis and interpretation. KMK material propagation.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kumar, V., Kim, S.H., Priatama, R.A. et al. NH4+ Suppresses NO3–-Dependent Lateral Root Growth and Alters Gene Expression and Gravity Response in OsAMT1 RNAi Mutants of Rice (Oryza sativa). J. Plant Biol. 63, 391–407 (2020). https://doi.org/10.1007/s12374-020-09263-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12374-020-09263-5