Abstract
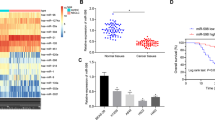
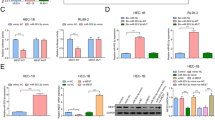
Cisplatin (DDP)-based strategies are the first-line treatment for cancers; however, resistance to DDP remains a major obstacle to cancer treatment. The current study set out to investigate the effects of microRNA-181c (miR-181c) on the resistance of ovarian cancer cells to DDP. Ovarian cancer-associated miRs as well as the target messenger RNAs were screened using microarray-based analysis followed by determining the expression patterns of miR-181c and mesoderm-specific transcript (MEST) in ovarian cancer tissues with RT-qPCR and Western blot analysis. Subsequently, dual-luciferase reporter gene assay was performed to confirm the targeting relation between miR-181c and MEST. Through gain- or loss-of-function experiments, the study explored the mechanism by which miR-181 regulated MEST to influence the resistance of ovarian cancer cells to DDP via the Wnt/β-catenin signaling pathway. Afterwards, extracellular vesicles (EVs) were isolated from bone marrow stromal cells (BMSCs) and co-cultured with ovarian cancer cells to further investigate the effects of overexpressed miR-181 delivered by BMSCs-derived EVs on ovarian cancer cell resistance to DDP. miR-181c was significantly downregulated, while MEST was up-regulated in ovarian cancer. miR-181c was verified to specifically bind to MEST. Overexpressed miR-181c depleted the expression of MEST to attenuate the resistance of ovarian cancer cells to DDP by inactivating the Wnt/β-catenin signaling pathway. Furthermore, the delivery of overexpressed miR-181c by BMSCs-derived EVs was found to suppress the resistance of ovarian cancer cells to DDP. These findings demonstrate that miR-181c delivered by BMSCs-derived EVs down-regulates MEST, to inactivate the Wnt/β-catenin signaling pathway, thus repressing the resistance of ovarian cancer cells to DDP.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The datasets generated/analyzed during the current study are available.
References
Doubeni CA, Doubeni AR, Myers AE. Diagnosis and management of ovarian cancer. Am Fam Physician 2016;93:937–944.
Torre LA, et al. Ovarian cancer statistics, 2018. CA Cancer J Clin 2018;68:284–296.
La Vecchia C. Ovarian cancer: epidemiology and risk factors. Eur J Cancer Prev 2017;26:55–62.
Wang J, Wu GS. Role of autophagy in cisplatin resistance in ovarian cancer cells. J Biol Chem 2014;289:17163–17173.
Misganaw B, et al. Optimized prediction of extreme treatment outcomes in ovarian cancer. Cancer Inform 2015;14:45–55.
Natanzon Y, Goode EL, Cunningham JM. Epigenetics in ovarian cancer. Semin Cancer Biol 2018;51:160–169.
Becker A, et al. Extracellular vesicles in cancer: Cell-to-cell mediators of metastasis. Cancer Cell 2016;30:836–848.
Soekmadji C, Nelson CC. The emerging role of extracellular vesicle-mediated drug resistance in cancers: Implications in advanced prostate cancer. Biomed Res Int 2015;2015:454837.
Vakhshiteh F, Atyabi F, Ostad SN. Mesenchymal stem cell exosomes: A two-edged sword in cancer therapy. Int J Nanomed 2019;14:2847–2859.
Whiteside TL. Exosome and mesenchymal stem cell cross-talk in the tumor microenvironment. Semin Immunol 2018;35:69–79.
Wang J, et al. Bone marrow stromal cell-derived exosomes as communicators in drug resistance in multiple myeloma cells. Blood 2014;124:555–566.
Pal MK, et al. Microrna: A new and promising potential biomarker for diagnosis and prognosis of ovarian cancer. Cancer Biol Med 2015;12:328–341.
Liu M, et al. Microrna-mrna functional pairs for cisplatin resistance in ovarian cancer cells. Chin J Cancer 2014;33:285–294.
Yao L, et al. Mir181c inhibits ovarian cancer metastasis and progression by targeting prkcd expression. Int J Clin Exp Med 2015;8:15198–15205.
Zhao L, et al. Upregulation of mir-181c inhibits chemoresistance by targeting st8sia4 in chronic myelocytic leukemia. Oncotarget 2016;7:60074–60086.
Li X, et al. Isoform-specific imprinting of the mest gene in porcine parthenogenetic fetuses. Gene 2015;558:287–290.
Jung H, Lee SK, Jho EH. Mest/peg1 inhibits wnt signalling through regulation of lrp6 glycosylation. Biochem J 2011;436:263–269.
Li W, Zhu C, Li Y, Wu Q, Gao R. Mest attenuates ccl4-induced liver fibrosis in rats by inhibiting the wnt/beta-catenin signaling pathway. Gut Liver 2014;8:282–291.
Peng Y, Zhang X, Feng X, Fan X, Jin Z. The crosstalk between micrornas and the wnt/beta-catenin signaling pathway in cancer. Oncotarget 2017;8:14089–14106.
Arend RC, Londono-Joshi AI, Straughn JM Jr., Buchsbaum DJ. The wnt/beta-catenin pathway in ovarian cancer: a review. Gynecol Oncol 2013;131:772–779.
Zhang G, et al. The anti-oxidative role of micro-vesicles derived from human wharton-jelly mesenchymal stromal cells through nox2/gp91(phox) suppression in alleviating renal ischemia-reperfusion injury in rats. PLoS ONE 2014;9:e92129.
Kumar B, et al. Acute myeloid leukemia transforms the bone marrow niche into a leukemia-permissive microenvironment through exosome secretion. Leukemia 2018;32:575–587.
Ayuk SM, Abrahamse H, Houreld NN. The role of photobiomodulation on gene expression of cell adhesion molecules in diabetic wounded fibroblasts in vitro. J Photochem Photobiol B 2016;161:368–374.
Garces AH, Dias MS, Paulino E, Ferreira CG, de Melo AC. Treatment of ovarian cancer beyond chemotherapy: Are we hitting the target? Cancer Chemother Pharmacol 2015;75:221–234.
Galluzzi L, et al. Molecular mechanisms of cisplatin resistance. Oncogene 2012;31:1869–1883.
Zabaglia LM, et al. Decreased microrna mir-181c expression associated with gastric cancer. J Gastrointest Cancer 2018;49:97–101.
Liu J, Xing Y, Rong L. Mir-181 regulates cisplatin-resistant non-small cell lung cancer via downregulation of autophagy through the pten/pi3k/akt pathway. Oncol Rep. 2018;39:1631–1639.
Liu HN, Qie P, Yang G, Song YB. Mir-181b inhibits chemoresistance in cisplatin-resistant h446 small cell lung cancer cells by targeting bcl-2. Arch Med Sci 2018;14:745–751.
Zhao J, Nie Y, Wang H, Lin Y. Mir-181a suppresses autophagy and sensitizes gastric cancer cells to cisplatin. Gene 2016;576:828–833.
Chen L, et al. Zfp57 suppress proliferation of breast cancer cells through down-regulation of mest-mediated wnt/beta-catenin signalling pathway. Cell Death Dis 2019;10:169.
Kadota Y, et al. Gene expression of mesoderm-specific transcript is upregulated as preadipocytes differentiate to adipocytes in vitro. J Physiol Sci 2012;62:403–411.
Zeng B, Zhou M, Wu H, Xiong Z. Spp1 promotes ovarian cancer progression via integrin beta1/fak/akt signaling pathway. Onco Targets Ther 2018;11:1333–1343.
Hashimoto Y, Akiyama Y, Yuasa Y. Multiple-to-multiple relationships between micrornas and target genes in gastric cancer. PLoS ONE 2013;8:e62589.
Li X, et al. Exosome derived from human umbilical cord mesenchymal stem cell mediates mir-181c attenuating burn-induced excessive inflammation. EBioMedicine 2016;8:72–82.
Chen L, et al. Bmscs-derived mir-223-containing exosomes contribute to liver protection in experimental autoimmune hepatitis. Mol Immunol 2018;93:38–46.
Yu PN, et al. Downregulation of mir-29 contributes to cisplatin resistance of ovarian cancer cells. Int J Cancer 2014;134:542–551.
Chen QY, et al. Mir-206 regulates cisplatin resistance and emt in human lung adenocarcinoma cells partly by targeting met. Oncotarget 2016;7:24510–24526.
Acknowledgements
The authors would like to acknowledge the helpful comments on this paper received from the reviewers.
Author information
Authors and Affiliations
Contributions
L.Z. and M.D. designed the study. Z.R. and L.L. collated the data, carried out data analyses and produced the initial draft of the manuscript. L.Z. and M.D. contributed to draft the manuscript. All authors have read and approved the final submitted manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval and consent to participate
The experimental design for this study was approved by the Ethics Committee and Experimental Animal Ethics Committee of Shanghai Ninth People’s Hospital, School of Medicine, Shanghai Jiaotong University. Written informed consent was obtained from all participant or their relatives. The animal experiment strictly adhered to the principle to minimize the pain, suffering, and discomfort to experimental animals.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Ruan, Z., Lu, L., Zhang, L. et al. Bone marrow stromal cells-derived microRNA-181-containing extracellular vesicles inhibit ovarian cancer cell chemoresistance by downregulating MEST via the Wnt/β-catenin signaling pathway. Cancer Gene Ther 28, 785–798 (2021). https://doi.org/10.1038/s41417-020-0195-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41417-020-0195-6
This article is cited by
-
miR-106a-5p carried by tumor-derived extracellular vesicles promotes the invasion and metastasis of ovarian cancer by targeting KLF6
Clinical & Experimental Metastasis (2022)
-
Long non-coding RNA NEAT1 facilitates the growth, migration, and invasion of ovarian cancer cells via the let-7 g/MEST/ATGL axis
Cancer Cell International (2021)
-
RPN2 is targeted by miR-181c and mediates glioma progression and temozolomide sensitivity via the wnt/β-catenin signaling pathway
Cell Death & Disease (2020)