Endothelial-Derived Interleukin-1α Activates Innate Immunity by Promoting the Bactericidal Activity of Transendothelial Neutrophils
- 1Beijing Traditional Chinese Veterinary Engineering Center and Beijing Key Laboratory of Traditional Chinese Veterinary Medicine, Beijing University of Agriculture, Beijing, China
- 2Department of Mechanics and Engineering Science, College of Engineering, Academy for Advanced Interdisciplinary Studies, and Beijing Advanced Innovation Center for Engineering Science and Emerging Technology, College of Engineering, Peking University, Beijing, China
- 3Beijing Advanced Innovation Center for Food Nutrition and Human Health, College of Veterinary Medicine, China Agricultural University, Beijing, China
Migration of neutrophils across endothelial barriers to capture and eliminate bacteria is served as the first line of innate immunity. Bacterial virulence factors damage endothelium to produce inflammatory cytokines interacts with neutrophils. However, the mechanisms that behind endothelial-neutrophil interaction impact on the bactericidal activity remain unclear. Therefore, we aimed to find the target proteins on endothelial cells that triggered the bactericidal activity of transendothelial neutrophils. Herein, we built the infected models on rats and endothelial-neutrophil co-cultural system (Transwell) and discovered that endothelial-derived IL-1α promoted the survival of rats under Escherichia coli infection and enhanced the bactericidal activity of transendothelial neutrophils in vivo and in vitro. Results further showed that IL-1α was inhibited by lipopolysaccharide (LPS) in the endothelial-neutrophil interaction. We found that LPS mainly damaged cell membrane and induced cell necrosis to interrupt neutrophil migration from endothelial barrier. Thus, we used the isobaric tags for relative and absolute quantification (iTRAQ) method to identify different proteins of endothelial cells. Results showed that IL-1α targeted cellular plasma membrane, endoplasmic reticulum and mitochondrial envelope and triggered eleven common proteins to persistently regulate. During the early phase, IL-1α triggered the upregulation of cell adhesion molecules (CAMs) to promote neutrophil adhesion, while oxidative phosphorylation was involved in long time regulation to induce transmigration of neutrophils against bacteria. Our results highlight the critical mechanism of endothelial-derived IL-1α on promoting bactericidal activity of transendothelial neutrophils and the findings of IL-1α triggered proteins provide the potentially important targets on the regulation of innate immunity.
Introduction
Endothelial cells are the inner cell lines connected with immune cells and epithelium (Rohlenova et al., 2018). One kind of immune cells, neutrophils, must across endothelial cells to reach the infected sites against pathogenic infection (Papayannopoulos, 2018). In turn, bacteria employ their virulence factors to hijack endothelial cells and induce inflammatory cytokine release as the major strategy to break through epithelium barrier and inhibit innate immune system (Liu et al., 2017; Yuan et al., 2018). For instance, the lipopolysaccharide (LPS) secreted from Escherichia coli (E. coli) impacts the release of inflammatory mediators to regulate the progress of infection by leading immune cell damage (Li et al., 2016; Presicce et al., 2020). It worth to note that the inflammatory cytokine, interleukin-1α (IL-1α) can induce neutrophil extracellular traps (NETs) to activity endothelial cell (Folco et al., 2018). As similar as our previous research illustrated that endothelial IL-1α enhanced the bacterial killing of transendothelial neutrophils (Liu et al., 2016). In addition, IL-1α is primarily associated with inflammatory during the pathogenesis induced by bacterial infection (Dinarello, 2011; Menghini et al., 2019). However, the mechanisms of how endothelial-derived IL-1α regulate the killing ability of transendothelial neutrophils remain unknown. Thus, we hypothesized that endothelial IL-1α modulated endothelial cells to impact bacterial killing of transendothelial neutrophils.
In this work, we aimed to investigate how endothelial-derived IL-1α impacted the bactericidal activity of transendothelial neutrophils during endothelial-neutrophil interaction though two E. coli infected models of rats and endothelial-neutrophil co-cultural system (Transwell). Further, we intended to find the regulated difference proteins on endothelial cells that triggered by IL-1α via using iTRAQ-based quantitative proteomics.
Materials and Methods
Animals
Rats (1-day rats and 1–2-month rats) were purchased from academy of military medical sciences, Beijing, China (Certificate Number: SCXK-PLA 2012-0004). One day rats were obtained to isolate primary RIMVECs and 1–2-months rats were used for the rat infection.
Ethics Statement
The experimental protocols involving rats were gained an approval by the Institutional Animal Care and Use Committee of the Academy of Military Medical Sciences (Beijing, China; approval no. SYXK2014-0002).
Rat Infection
Rats (1–2 month, about 500 g, 10 rats per group) were infected with 109 colony-forming units (CFUs) of E. coli (serotype O55:B5) orally. To simulate the situation of stress-induced LPS accumulation. We set up the group of additional LPS by adding 1 μg/g of LPS (from E. coli serotype O55:B5, Sigma-Aldrich) mixed with E. coli suspension. After 24 h infection, IL-1α, IL-1β, IL-6, intercellular adhesion molecule-1 (ICAM-1) and Tumor Necrosis Factor (TNF-α) from rat serums were detected by the ELISA kits (BD Biosciences) according to the instructions. For further investigating the survival of E. coli infected rats, simultaneous addition of IL-1α (rat recombinant, Sigma-Aldrich) with 10 ng/g for each infected group. Then the ratios of rat survival were recorded. Lastly the E. coli that survived in rat colons were detected by the colony count technique (colony-forming units, CFUs).
Primary Endothelial Cell Culture
Primary rat intestinal mucosal microvascular endothelial cells (RIMVECs) were separated from the colons of 1 day-rats and then cultured in complete Dulbecco’s modified eagle medium (DMEM, Gibco) containing 2 mM L-glutamic acid, 50 mg/l gentamycin, 100 U/mL penicillin/streptomycin and 20% heat-inactivated fetal bovine serum (FBS, Gibco). The identification of RIMVECs was obtained as previous protocol (Liu et al., 2016).
Isolation of Blood Neutrophils
Rat fresh neutrophils were isolated from heparinized whole blood of healthy rats by gradient centrifugation assay using Percoll reagent (GE Healthcare) as previous published methods (Liu et al., 2016). Then neutrophils were washed with HBSS and preserved in RPMI-1640 medium (Gibco) for later use after counting and viability assessment.
Detecting the Damage of LPS on RIMVECs
RIMVECs (1 × 104 cells/well) were seeded in a 96-well plate and treated with a final concentrations of 1 μg/mL LPS for different time points (0.5, 1, 2, 4, 8, 12, and 24 h) at 37°C in a 5% CO2 atmosphere. After treatment, the cytotoxicity of RIMVECs was detected by 10 μL of WST-1 reagents (Roche). After 1 h incubation at 37°C, the absorbance was detected by a fluorescence microplate reader (Life Science & Technology) at wavelength of 450 nm. The percentage of RIMVECs survival was calculated based on the ratio of absorbance compared to DMEM treated group. After RIMVECs treated with LPS, then cells were washed with PBS and incubated with PI (5 μg/mL, Sigma-Aldrich) for 30 min. The PI positive cells presented the membrane damaged cells and fluorescence intensity of PI was immediately detected with excitation wavelength at 535 nm and emission wavelength at 615 nm.
Flow Cytometry
To record the proportion of necrosis and apoptosis on RIMVECs leaded by LPS, we used an Annexin-V-FITC (Annexin-V-fluorescein isothiocyanate) and propidium iodide (PI) double staining kit (B&D system) to track the cytotoxicity of LPS. Annexin-V was employed to label membrane phosphatidylserine on the surface of early apoptotic cells, which displayed green fluorescence due to FITC. PI was used to sort the necrotic cells by further binding to cellular DNA and showing red fluorescence. Detection and analysis of necrosis were used BD FACSAriaTM flow cytometry and FACSDiva software (BD Biosciences) based as our previous publish method (Liu et al., 2017).
Infection of the Endothelial-Neutrophil Interaction
RIMVECs (1 × 104 cells/well) were seeded onto the 5.0 μm pore size polycarbonate resin transwell membranes to reach confluence and form a monolayer on the upper chamber of transwell system (Corning) and measured by TEER using the Millicell Electrical Resistance System (ERS)-2 (EMD Millipore, Billerica, United States). Then fresh neutrophils were added into the upper chambers and E. coli in the bottom chambers as illustrated in Figure 1E. All cells were cultured in a DMEM medium (Gibico) supplemented with 10% FBS at 37°C in a 5% CO2 atmosphere. Images of RIMVECs and neutrophils were captured by a confocal microscopy (Leica, SP8). Then E. coli were infected with transendothelial neutrophils at the bottom chambers of transwell for 4 h. IL-1α or LPS were added in the co-culture medium at the final concentration of 1 μg/mL or 10 ng/mL, respectively. Lastly, both the extracellular and intracellular E. coli (neutrophils, 5 × 108 cells) were collected to calculate using the colony count technique (CFUs) according to previous study (Liu et al., 2016).
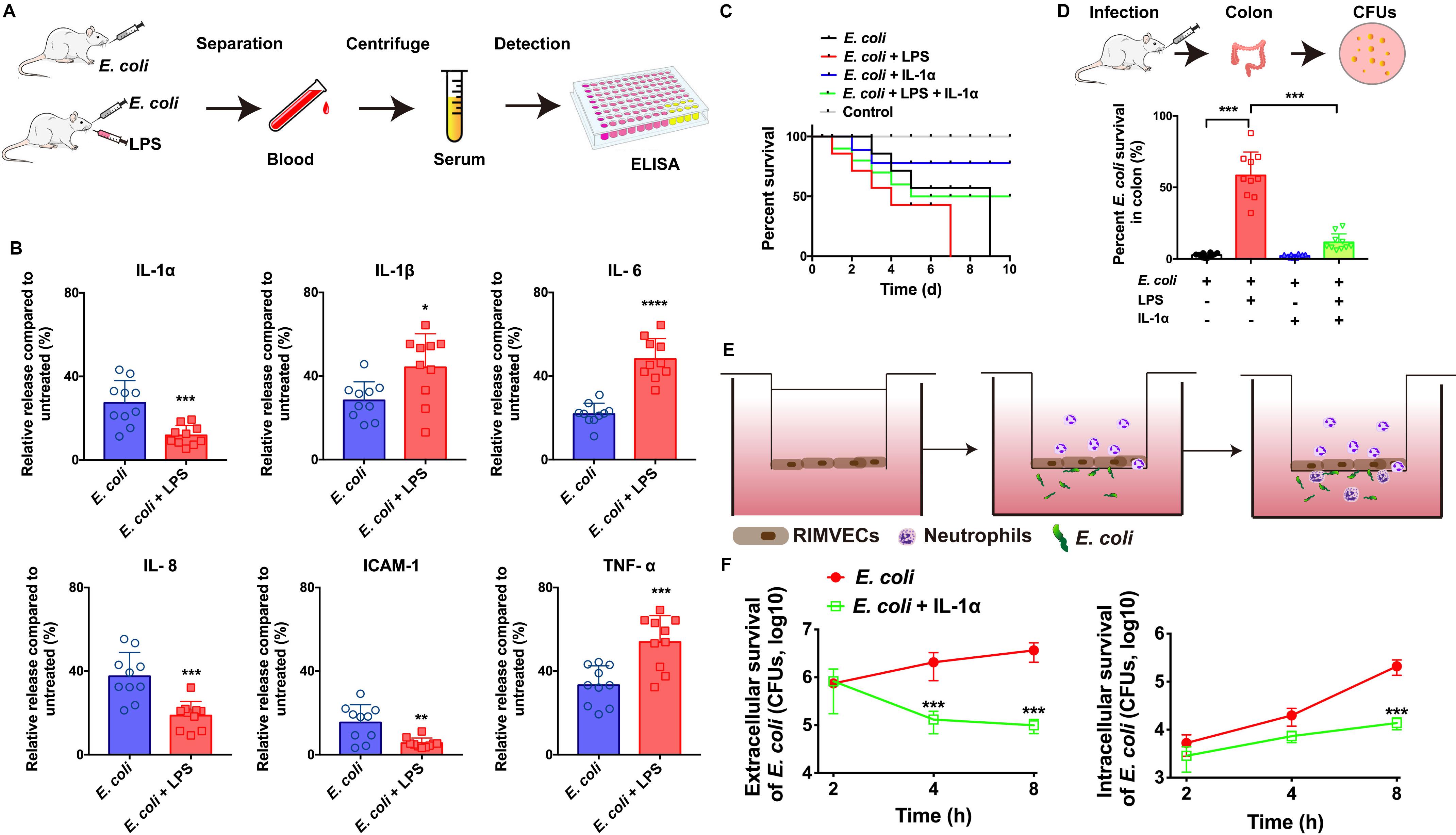
Figure 1. IL-1α facilitated the bacterial killing on the in vivo and in vitro model of E. coli infection. (A) E. coli infection in vivo rat model workflow. (B) IL-1α, IL-1β, IL-6, IL-8, ICAM-1, and TNF-α were detected by ELISA. Rats were infected by E. coli (109 CFU/g) with or without LPS (1 μg/mL) treatment. After 24 h infection, the serum of every rats including both dead or live rats were collected to detect the inflammation-associated cytokines by ELISA. (C) IL-1α increase the survival of LPS-induced rat death. A total of 109 CFU/g E. coli were infected with rats for 24 h. LPS (1 μg/g) or IL-1α (10 ng/g) were treated with the infected rats. The ratios of rat survival were determined from 10 days’ treatment. Gray line showed the percent survival of uninfected rats, red line represented the E. coli infection, blue line was the LPS treatment of E. coli infection, green line represented LPS plus IL-1α treated with E. coli infected rats. (D) IL-1α decreased the bacterial load in rat colon. The number of bacteria was counted by the colony count technique (CFU). Percentage of E. coli were compared to untreated group. Data are shown as means ± SD (*P < 0.05; **P < 0.01; ***P < 0.001, n = 10). (E) Scheme of co-culture system for RIMVECs-neutrophils interactions in vitro. RIMVECs were seeded on upper chambers for 24 h to form monolayer. Then neutrophils were added in the upper chambers. E. coli infected (F) IL-1α increased the transendothelial neutrophils killing. E. coli infected in bottom chambers treated for different time points (2, 4, and 8 h) and IL-1α (10 ng per well) was also treated in the upper chambers. Both the extracellular and intracellular bacteria in neutrophils (5 × 108 cells) were used to count the E. coli CFUs. The red lines represented E. coli infection and green lines showed the IL-1α treatment of E. coli infection. Values represent the mean ± SD (***P < 0.001, n = 6).
Western Blot
RIMVECs were collected from the transwell system. RIMVECs were lysed in 1 mL of RIPA lysis buffer with 10 μL phenylmethanesulfonyl fluoride (PMSF, 1 mmol/L, Beyotime) on the ice for 20 min. Then the cell lysates were used to gain the whole proteins by centrifugation at 15,000 rpm for 12 min and proteins were quantified by the BCA method (Pierce). Fifty microgram of proteins were used to detect the expression of IL-1α by Western Blot assay. Briefly, separation of proteins used SDS-PAGE with 15% polyacrylamide gels and transferred onto a PVDF membranes (Beyotime). The primary antibody of rabbit anti- IL-1α (a dilution of 1: 1000, Invitrogen) was incubated with membranes at 4°C overnight and secondary antibody of goat anti-rabbit antibody (a dilution of 1: 3000, Beyotime) covered the membranes for 1 h at room temperature. Gray values of protein bands were quantified by ImageJ software.
LPS Detection
Concentrations of LPS were determined by a LAL (Limulus Amebocyte Lysate, LONZA) assay. Briefly, the samples including LPS were diluted in the free endotoxin water and detected by LAL reagent (sensitivity 0.125 EU/mL) as previous published protocols (Mitra et al., 2014). 2.5 EU of LPS approximated 1 ng.
Immunostaining and Confocal Microscopy
RIMVECs were seeded on glass coverslips (15 mm, NEST) in a 24-well plate and incubated with LPS for 4 h. Then cells were fixed by 4% paraformaldehyde and incubated with the primary antibodies, rabbit anti-IL-1α (a dilution of 1: 500, Invitrogen) at 4°C overnight. After PBS washed twice, cells were incubated with the FITC-labeled goat anti-rabbit IgG (H + L) (a dilution of 1: 1000, Beyotime) at 4°C for 2 h, which was used to visualize the IL-1α protein. Images were captured by the LAS AF Lite software (Leica).
Protein Preparation and iTRAQ Labeling
RIMVECs were treated with IL-1α (final concentration of 10 ng/mL) at 37°C incubated in a 5% CO2 atmosphere for 0, 2, 4, and 8 h. The proteins of RIMVCEs were extracted by RIPA lysate buffer (Beyotime) and quantified by a BSA kit (Beyotime). The concentrations of sample proteins were detailed in Supplementary Table S1. Two hundred microgram proteins were incubated in iTRAQ-4-plex kit (AB Sciex, PN: 4352135) proteolysis. The iTRAQ labeled, LC-MS/MS analysis and MALDI-TOF-TOF identifications were conducted by BIOMS company. Labels of 114, 115, 116, and 117 are represented 2, 4, 6, and 0 h treatment, respectively (Supplementary Table S1).
LC-MS/MS Analysis Based on TripleTOFTM 5600
The iTRAQ labeled samples were run though Durashell-C18 column (4.6 mm × 250 mm, 5 μm 100 AÅ, Agela, Catalog Number: DC952505-0) and dissolved in mobile phase A, which was 2% acetonitrile in water and phase B was 98% acetonitrile in water. The gradient elution program was: 0–5 min, 5% B; 5–35 min, 8% B; 35–62 min, 32% B; 62–64 min, 95% B; 64–68 min, 95% B; 68–72 min, 5% B. The injection volume was 3 μL and the flow rate was 0.7 mL/min. The parameters of mass spectrometry were: Ion spray voltage:2.3 kv; GS1:4; Curtain gas:35; DP:100; Top MS, m/z:350-1250; accumulation time: 0.25 s; product ion scan: IDA mumber:30; m/z:100-1500; accumulation time:0.1 s; Dynamic exclusion time: 25 s; Rolling CE: enabled; Adjust CE when using iTRAQ reagent: enabled; CES:5. Analysis of iTRAQ mass spectrometry by TripleTOFTM 5600 system using the software of ProteinPilot 4.0 (AB Sciex) and the database come from http://www.uniprot.org.
Data Availability
iTRAQ-based quantitative mass spectrometry proteomics data had been deposited to the ProteomeXchange Consortium via the PRIDE (Perez-Riverol et al., 2019) partner repository with the dataset identifier PXD019561.
Statistical Analysis
The significant differences between two groups were calculated using unpaired t-test with between two groups or one-way ANOVA among multiple groups and performed by GraphPad Prism 8.0 software. Results were expressed as means ± SD. Values are represented as column diagram (∗P < 0.05; ∗∗P < 0.01; ∗∗∗P < 0.001). All animals were used to analyze including both live and dead rats.
Results
IL-1α Prevented E. coli Infection in vivo and in vitro
To investigate whether IL-1α can impact on the innate immunity against bacterial infection, we firstly built the in vivo model of E. coli infected rats (Figure 1A) and the infection on co-culture system of neutrophils and endothelial cells in vitro (Figure 1E). We found that LPS of E. coli promoted the release of TNF-α, IL-6, and IL-1β in the serums of infected rats, while LPS suppressed IL-1α, IL-8, and ICAM-1 (Figure 1B). It suggested that LPS might damage endothelial-neutrophil interaction due to interrupt the inflammation. Since IL-1α can secrete from endothelial cells as well as the IL-8 and ICAM-1 are crucial for neutrophils recruitment (Gunther et al., 2017). Therefore, based on our previous study as well (Liu et al., 2016), we hypothesized that IL-1α acted as the important role on endothelial cells to activate the innate immunity. Next, results also confirmed IL-1α could increase the survival of LPS induced the E. coli infected rats (Figure 1C) and decrease the bacterial loading in rat colon (Figure 1D), suggesting that IL-1α could prevent the LPS induced bacterial expansion in vivo. For more clearing illustrated the function of IL-1α on endothelial-neutrophil interaction, we employed a Transwell system to co-culture of rat intestinal microvascular endothelial cells (RIMVECs) and neutrophils to evaluate the bacterial killing ability of transendothelial neutrophils. As Figure 1F showed, IL-1α treatment decreased both the intracellular and extracellular E. coli, suggesting that the bacterial killing activity of transendothelial neutrophils was enhanced by IL-1α.
Endothelial-Derived IL-1α Was Inhibited by LPS Though Damaging Endothelial Cells and Neutrophils
LPS from E. coli are frequently reported that impairs endothelial cells (Yang et al., 2016; Zhou et al., 2018; Huang et al., 2019). To investigate how LPS impacted RIMVECs, we utilized a double staining of PI and Annexin-V to sort the LPS treated RIMVECs by flow cytometry. As showed in Figure 2A, LPS induced RIMVECs necrosis in a dose dependent manner (4 h treatment) and further led cell death and impaired cellular membranes in a time dependent way (Figure 2B). It was consisted with previous reports that LPS injury tissue and led cell necroptosis (Li et al., 2016; Huang et al., 2019). In fact, LPS action on endothelial cells is intend to occur an inflammation within endothelial-neutrophil interactions (Al-Banna et al., 2013; Shi et al., 2014), and IL-1 family is closely related with inflammation (Dinarello, 2011; Liu et al., 2016; Gunther et al., 2017). In addition, we found that LPS damaged the transendothelial neutrophils and led the bacterial escape from cells (Figure 1C). Take it together, IL-1α prevented E. coli infection by enhancing the killing ability of transendothelial neutrophils, while LPS destroyed it. Therefore, we next detected the amount of LPS and IL-1α in the co-culture system to reveal the function of each other. We found that the expression of endothelial-derive IL-1α was inhibited by LPS (Figure 1D). Results also indicated that the release of LPS and IL-1α in co-culture transwell system was competitive (Figure 1E) and the addition of LPS did weaken the expression of IL-1α (Figure 1F). Altogether, IL-1α might be the key factor that impact bacterial killing during endothelial-neutrophil interaction.
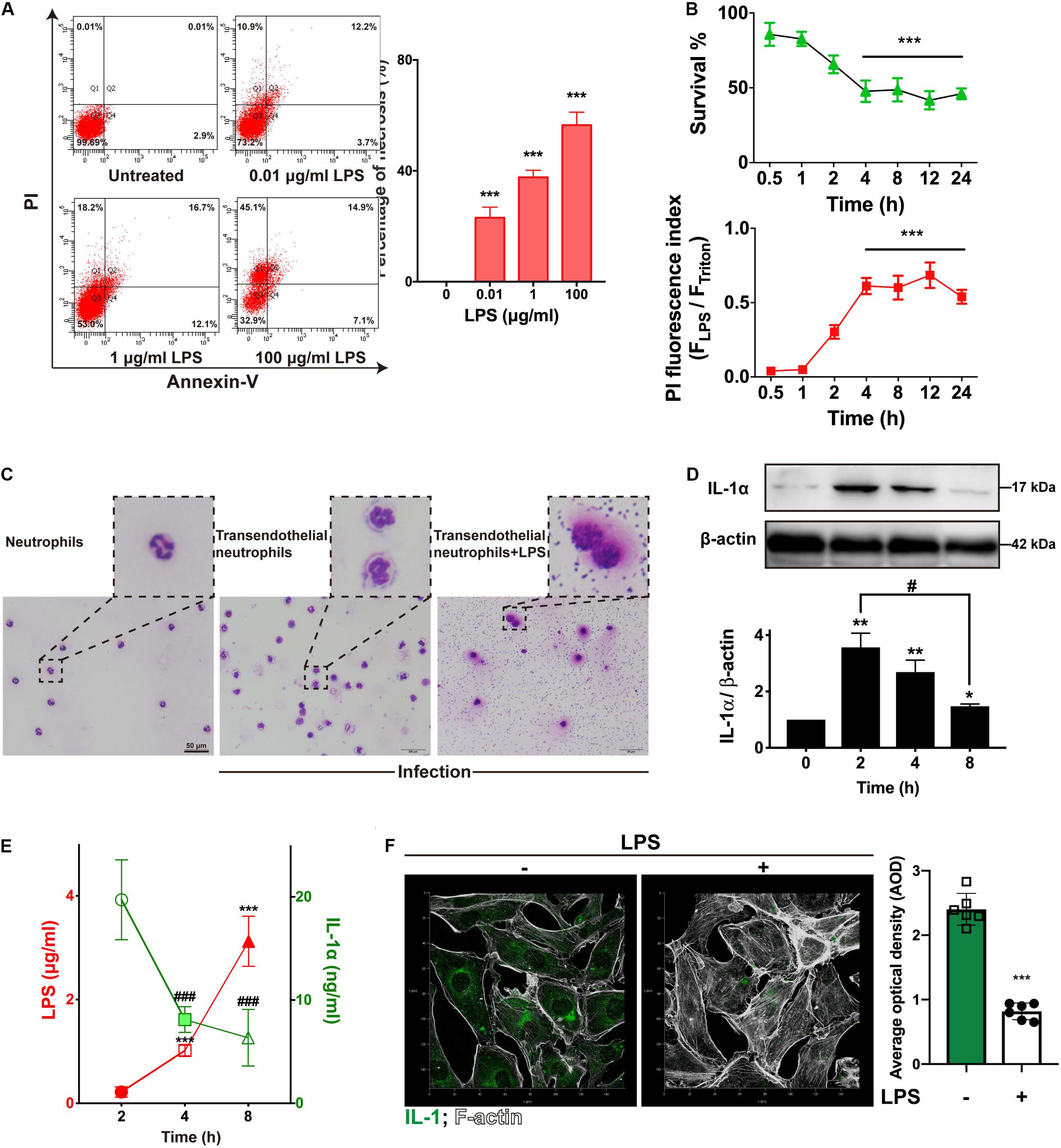
Figure 2. LPS damaged endothelial cells and neutrophils to suppress IL-1α. (A) LPS induced RIMVECs necrosis. Different concentrations of LPS (0–100 μg/mL) were treated RIMVECs for 4 h. Then cells were labeled by PI and Annexin-V staining. Apoptosis and necrosis of RIMVECs (at least 10,000 cells) were distinguished by flow cytometry assay. The percentage of Q1 and Q2 area were counted from A presented the necrotic RIMVECs. (B) LPS induced cytotoxicity of RIMVECs in a time dependent manner. LPS (1 μg/mL) treated RIMVECs for different time points (0.5–24 h). Then the survival of RIMVECs were determined by WST-1 assay. PI positive RIMVECs were recorded by a plate reader (SpectraMax M5) at the excitation wavelength of 535 nm and emission wavelength of 615 nm. The detached RIMVECs were also counted. (C) Transwell system were treated with 1 μg/mL LPS for 4 h. Transendothelial neutrophils were dyed with Switzerland staining. Images were captured by an optical microscope (Olympus). (D) The expression of IL-1α in co-culture system was decreased in a time dependent manner. (E) LPS suppressed the release of IL-1α. IL-1α release and concentrations of LPS were detected by ELISA or LAL (Limulus Amebocyte Lysate) assay, respectively. (F) The immunofluorescence of IL-1α in RIMVECs with or without LPS treatment. Values represent the mean ± SD (*P < 0.05; **P < 0.01; ***P < 0.001; ###P < 0.0001, #P < 0.05, n = 6 or three independent biological repetition).
IL-1α Facilitated Neutrophil Killing via Sustaining Oxidative Phosphorylation Activity
For deeply exploring the modulatory function of IL-1α, iTRAQ labeling technology combined with mass spectrometry proteomic analysis were used for investigating the differentially expressed proteins of RIMVECs from Transwell system (Figure 3A). We first analyzed the location of different proteins and found that most of them were disturbed on plasma membrane compared to 0 h (2 h about 33%, 4 h was 35% and 8 h was 40%, Figure 3B). Venn diagram showed that there had 31 proteins of upregulation and 29 proteins of downregulation at 2 h, for 4 h treatment, 14 upregulations, 19 downregulations and 31 upregulations, 26 downregulations at 8 h (Figure 3C). As showed in the heat map (Figure 2D), compared to untreated group, there are 63, 37, or 63 proteins significant regulation in 2, 4, or 8 h treatment, respectively (Supplementary Table S2). These data showed that IL-1α is a complex regulation in protein levels. Combating with our previous results that IL-1α enhanced both extracellular and intracellular bacterial killing of transendothelial neutrophils mainly focused on the long-time treatment (4–8 h, Figure 1F). Thus, we believe that the common regulated proteins in all time points is playing the key role. Therefore, we analyzed that 11 proteins were sustained to regulate significantly, among them 5 proteins upregulation and 5 proteins downregulation (Figure 3E). Interesting, one protein named Endoplasmin (ENPL) was upregulated at 2 h while downregulated at 4 and 8 h. All the information and analysis of the common regulated proteins were detailed in Table 1. Nevertheless, the function and location of ENPL still remained unclear. But it was worth noting that there were two proteins including ATPA and NDUS1 upregulated responded to oxidative phosphorylation.
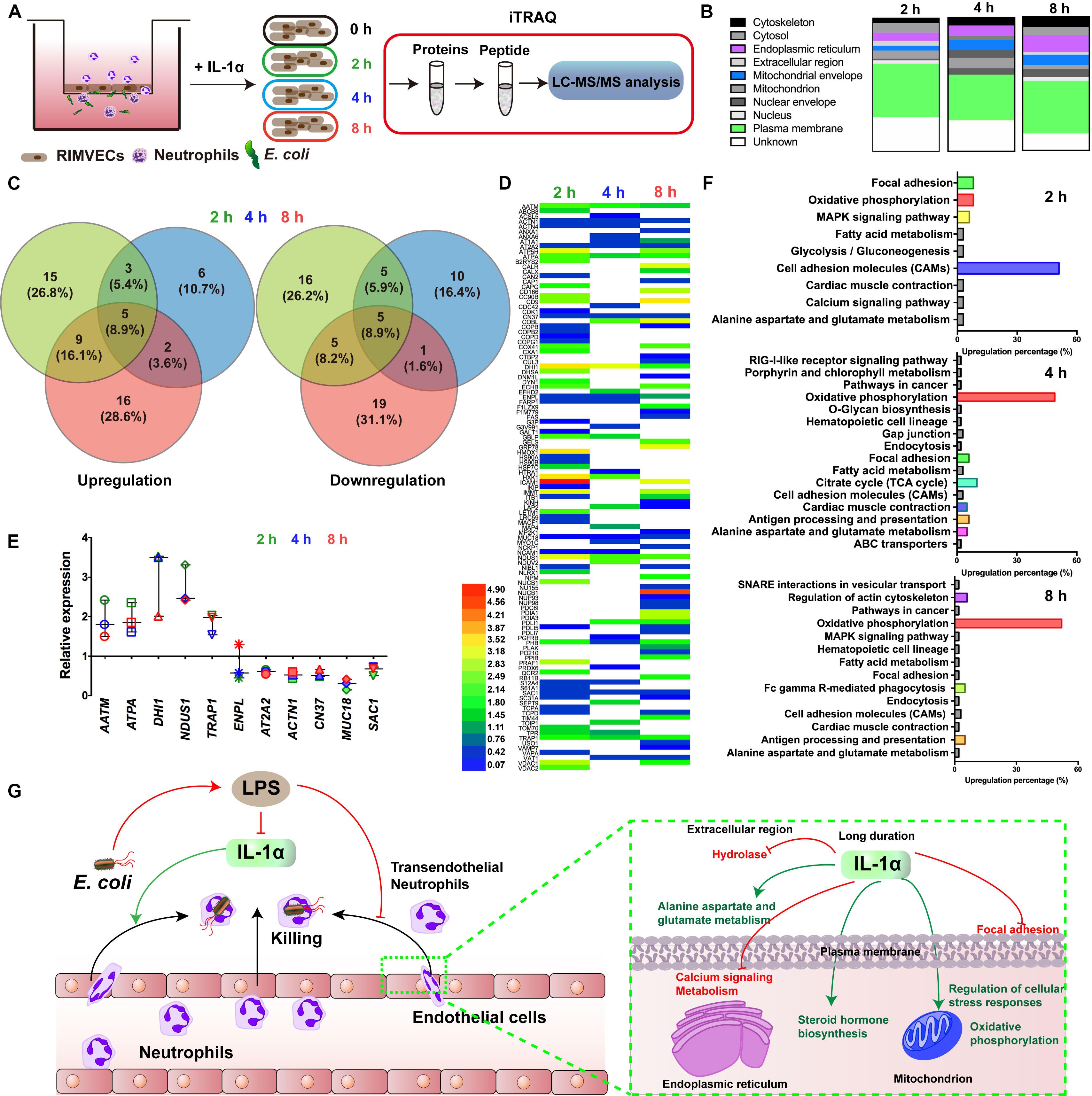
Figure 3. Oxidative phosphorylation was required for IL-1α activated-endothelial cells. (A) Scheme of the iTRAQ experimental set-ups. (B) The regulated proteins were mostly distributed on plasma membrane. RIMVECs were treated with IL-1α (10 ng/mL) for 2, 4, or 8 h and the proteins regulation of RIMVECs were analyzed by iTRAQ. (C) Venn diagram showed the percentage of upregulated and downregulated proteins, which were drawn by Venny 2.1.0 software online. (D) Heat map of the differential protein analyzed by iTRAQ. RIMVECs were treated with IL-1α (10 ng/mL) for 2, 4, or 8 h. At every time points, the proteins of RIMVECs were collected for iTRAQ assay. The image of heat map was made by Heml software. (E) The common expressed proteins of RIMVECs induced by IL-1α at all the treated times (n = 3, down-up regulated difference >1.5-fold change). (F) IL-1α upregulated the cell adhesion molecules (CAMs) of RIMVECs at 2 h treatment, while mainly triggers oxidative phosphorylation at 4 and 8 h. (G) Scheme of endothelial IL-1α facilitated transendothelial neutrophil killing.
Next, we deduced that oxidative phosphorylation was required for IL-1α-dependent activation of endothelial cells. Therefore, in order to more detail the functional regulation, we analyzed that the pathway cascade in 2, 4, and 8 h. As showed in Figure 3F, cell adhesion molecules (CAMs) major responded to 2 h treatment of IL-1α. It was consisted with our previous results that LPS targeted ICAM-1 and IL-1α to affect the bacterial killing of transendothelial neutrophils. As we proposed that the accumulation of IL-1α is the importance factor that activates the transendothelial neutrophils, we found that in long time treatment of IL-1α, oxidative phosphorylation is mainly regulated pathways (4 h was 49% and 8 h was 52%). Oxidative phosphorylation is vital for physiological function regulations in most eukaryocyte, especially in endothelial cells (Papa et al., 2012; Montorfano et al., 2014; Nath and Villadsen, 2015). We further found that the oxidative phosphorylation induced by IL-1α occurred on mitochondrial envelope (Table 1). Take it together, it revealed that additional IL-1α treated in the E. coli infected co-culture system of endothelial cells and neutrophils was originally enhanced the adhesion of neutrophils and then promoted the migration of neutrophils by triggering oxidative phosphorylation to generates ROS. It consisted with the fact that neutrophil transmigration across endothelial cells was essential for ROS and sustaining the inflammatory response (Mittal et al., 2017). Combining with these researches, our data further revealed a new function that IL-1α induced the oxidative phosphorylation of endothelial cells to assist transendothelial neutrophils killing.
Discussion
Endothelial cells not only form the physical barrier against pathogenic invasion (Sturtzel, 2017; He et al., 2020), but also have the regulation of transendothelial neutrophils. Indeed, endothelial cells turn into the activated form during the migration of neutrophils (Mittal et al., 2017; Folco et al., 2018). The action of transendothelial migration is a series of complex physical and biological processes (Kolaczkowska and Kubes, 2013; Mooren et al., 2014). In general, bacterial infection is connected with endothelial cell activation and neutrophil migration. Many bacterial pathogens utilize their toxins to disrupt endothelial integrity and inhibited immune cells as confirmed as our results (Figure 2) and further trigger the inflammation (Kim et al., 2010; Amedei and Morbidelli, 2019; Le Guennec et al., 2020). However, we do not know the role of endothelial cells act on the transendothelial migration during innate immunity. In this study, we focused on endothelial-derived IL-1α, because accumulation of IL-1α release from endothelial cells is the signaling for transendothelial migration of neutrophils (Burzynski et al., 2015). Our previous results also showed that IL-1α activated endothelial cells to promote neutrophil killing by improving the lysozyme activation (Liu et al., 2016). In this paper, we also found that IL-1α promoted bacterial killing of transendothelial neutrophils (Figure 1F). More interesting, LPS inhibited the release of IL-1α in vivo and in vitro (Figures 1, 2). It indicated that LPS damaged endothelial cells and interrupted the bacterial killing of transendothelial neutrophils was connected with IL-1α. Unlike other researches show that IL-1α has the ability to induce neutrophil-endothelial cell adhesion (Macmillan et al., 2010), these findings performed a novel link of IL-1α on the bacterial killing activity within transendothelial migration.
IL-1α modulates both endothelial cells and neutrophils, involving inflammation (Dinarello, 2011; Burzynski et al., 2015; Altmeier et al., 2016). Endothelial cells are the center role involved in neutrophils and inflammation (Sturtzel, 2017; Rohlenova et al., 2018). Therefore, we targeted on the different proteins of endothelial cells by IL-1α treatment. It worth to note that IL-1α have a specific time dependent function on protein regulations of endothelial cells. In the early state, IL-1α might promote neutrophil recruitment by upregulating ICAM, while oxidative phosphorylation was continuously required in the later stages (Figure 3F). These results had the important significance, it well explained that the generation of ROS was continuously required during endothelial-neutrophil interaction and inflammation (Papa et al., 2012; Montorfano et al., 2014; Mittal et al., 2017; Xu et al., 2019). More importantly, we also selected for specific functional proteins in detail (Figure 3D and Table 1), we believed these proteins would useful for further study of IL-1α on bactericidal activity of transendothelial neutrophils.
Conclusion
We demonstrated that endothelial-derived IL-1α was critical for neutrophil killing during endothelial-neutrophil interaction. As illustrated in Figure 3G, bacterial LPS inhibited the release of IL-1α from endothelial cells and further prevented the bacterial killing ability of transendothelial neutrophils. In turn, IL-1α was utilized as the signaling to trigger the transendothelial neutrophils killing. iTRAQ-based quantitative mass spectrometry proteomic analysis illustrated that IL-1α inhibited the hydrolase in the extracellular region, focal adhesion of plasma membrane and calcium signaling metabolism on endoplasmic reticulum. IL-1α promoted alanine aspartate and glutamate metabolism on plasma membrane, enhanced steroid hormone biosynthesis on endoplasmic reticulum and also increased the regulation of cellular stress responses and oxidative phosphorylation on mitochondrion.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/ Supplementary Material.
Ethics Statement
The animal study was reviewed and approved by the experimental protocols involving rats were gained an approval (SCXK, 2016-006). All animals were approved by the Genentech Institutional Animal Care and Use Committee at the China Agricultural University (SYXK, 2016-0008).
Author Contributions
HD and GH conceived the project. XL, XM, and HD did the research design. XL, HZ, and SH performed the experiments. XL, GH, and HD did the data analysis and wrote the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the National Natural Science Foundation of China (Grant No. 3172558) and China’s 13th Five-Year plan (2017YFD0501501).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcell.2020.00590/full#supplementary-material
Abbreviations
E. coli, Escherichia coli; IL-1 α, Interleukin-1 α; LPS, Lipopolysaccharide; RIMVECs, rat intestinal mucosa microvascular endothelial cells; iTRAQ, isobaric tags for relative and absolute quantification.
References
Al-Banna, N. A., Toguri, J. T., Kelly, M. E., and Lehmann, C. (2013). Leukocyte-endothelial interactions within the ocular microcirculation in inflammation and infection. Clin. Hemorheol. Microcirc. 55, 423–443. doi: 10.3233/ch-131780
Altmeier, S., Toska, A., Sparber, F., Teijeira, A., Halin, C., and Leibundgut-Landmann, S. (2016). IL-1 coordinates the neutrophil response to C. albicans in the oral mucosa. PLoS Pathog. 12:e1005882. doi: 10.1371/journal.ppat.1005882
Amedei, A., and Morbidelli, L. (2019). Circulating metabolites originating from gut microbiota control endothelial cell function. Molecules 24:3992. doi: 10.3390/molecules24213992
Burzynski, L. C., Humphry, M., Bennett, M. R., and Clarke, M. C. (2015). Interleukin-1alpha activity in necrotic endothelial cells is controlled by caspase-1 cleavage of interleukin-1 receptor-2: implications for allograft rejection. J. Biol. Chem. 290, 25188–25196. doi: 10.1074/jbc.m115.667915
Dinarello, C. A. (2011). Interleukin-1 in the pathogenesis and treatment of inflammatory diseases. Blood 117, 3720–3732. doi: 10.1182/blood-2010-07-273417
Folco, E. J., Mawson, T. L., Vromman, A., Bernardes-Souza, B., Franck, G., Persson, O., et al. (2018). Neutrophil extracellular traps induce endothelial cell activation and tissue factor production through interleukin-1alpha and cathepsin G. Arterioscler. Thromb. Vasc. Biol. 38, 1901–1912. doi: 10.1161/atvbaha.118.311150
Gunther, S., Deredge, D., Bowers, A. L., Luchini, A., Bonsor, D. A., Beadenkopf, R., et al. (2017). IL-1 Family cytokines use distinct molecular mechanisms to signal through their shared co-receptor. Immunity 47, 510–523.e514.
He, M., Martin, M., Marin, T., Chen, Z., and Gongol, B. (2020). Endothelial mechanobiology. APL Bioeng. 4:10904.
Huang, X., Zhu, J., Jiang, Y., Xu, C., Lv, Q., Yu, D., et al. (2019). SU5416 attenuated lipopolysaccharide-induced acute lung injury in mice by modulating properties of vascular endothelial cells. Drug Des. Devel. Ther. 13, 1763–1772. doi: 10.2147/dddt.s188858
Kim, M., Ashida, H., Ogawa, M., Yoshikawa, Y., Mimuro, H., and Sasakawa, C. (2010). Bacterial interactions with the host epithelium. Cell Host. Microbe. 8, 20–35. doi: 10.1016/j.chom.2010.06.006
Kolaczkowska, E., and Kubes, P. (2013). Neutrophil recruitment and function in health and inflammation. Nat. Rev. Immunol. 13, 159–175. doi: 10.1038/nri3399
Le Guennec, L., Coureuil, M., Nassif, X., and Bourdoulous, S. (2020). Strategies used by bacterial pathogens to cross the blood-brain barrier. Cell Microbiol. 22:e13132.
Li, Z., Scott, M. J., Fan, E. K., Li, Y., Liu, J., Xiao, G., et al. (2016). Tissue damage negatively regulates LPS-induced macrophage necroptosis. Cell Death Differ. 23, 1428–1447. doi: 10.1038/cdd.2016.21
Liu, X., Ding, S., Shi, P., Dietrich, R., Martlbauer, E., and Zhu, K. (2017). Non-hemolytic enterotoxin of Bacillus cereus induces apoptosis in Vero cells. Cell Microbiol. 19:e12684. doi: 10.1111/cmi.12684
Liu, X., Dong, H., Wang, M., Gao, Y., Zhang, T., Hu, G., et al. (2016). IL-1alpha-induced microvascular endothelial cells promote neutrophil killing by increasing MMP-9 concentration and lysozyme activity. Immunol. Res. 64, 133–142. doi: 10.1007/s12026-015-8731-4
Macmillan, H. F., Rowter, D., Lee, T., and Issekutz, A. C. (2010). Intravenous immunoglobulin G selectively inhibits IL-1α-induced neutrophil-endothelial cell adhesion. Autoimmunity 43, 619–627. doi: 10.3109/08916931003599062
Menghini, P., Corridoni, D., Butto, L. F., Osme, A., Shivaswamy, S., Lam, M., et al. (2019). Neutralization of IL-1alpha ameliorates Crohn’s disease-like ileitis by functional alterations of the gut microbiome. Proc. Natl. Acad. Sci. U.S.A. 16, 26717–26726. doi: 10.1073/pnas.1915043116
Mitra, A., Joshi, S., Arjun, C., Kulkarni, S., and Rajan, R. (2014). Limulus amebocyte lysate testing: adapting it for determination of bacterial endotoxin in 99mTc-labeled radiopharmaceuticals at a hospital radiopharmacy. J. Nucl. Med. Technol. 42, 278–282. doi: 10.2967/jnmt.114.146779
Mittal, M., Nepal, S., Tsukasaki, Y., Hecquet, C. M., Soni, D., Rehman, J., et al. (2017). Neutrophil activation of endothelial cell-expressed TRPM2 mediates transendothelial neutrophil migration and vascular injury. Circ. Res. 121, 1081–1091. doi: 10.1161/circresaha.117.311747
Montorfano, I., Becerra, A., Cerro, R., Echeverria, C., Saez, E., Morales, M. G., et al. (2014). Oxidative stress mediates the conversion of endothelial cells into myofibroblasts via a TGF-beta1 and TGF-beta2-dependent pathway. Lab. Invest. 94, 1068–1082. doi: 10.1038/labinvest.2014.100
Mooren, O. L., Li, J., Nawas, J., and Cooper, J. A. (2014). Endothelial cells use dynamic actin to facilitate lymphocyte transendothelial migration and maintain the monolayer barrier. Mol. Biol. Cell 25, 4115–4129. doi: 10.1091/mbc.e14-05-0976
Nath, S., and Villadsen, J. (2015). Oxidative phosphorylation revisited. Biotechnol. Bioeng. 112, 429–437. doi: 10.1002/bit.25492
Papa, S., Martino, P. L., Capitanio, G., Gaballo, A., De Rasmo, D., Signorile, A., et al. (2012). The oxidative phosphorylation system in mammalian mitochondria. Adv. Exp. Med. Biol. 942, 3–37.
Papayannopoulos, V. (2018). Neutrophil extracellular traps in immunity and disease. Nat. Rev. Immunol 18, 134–147. doi: 10.1038/nri.2017.105
Perez-Riverol, Y., Csordas, A., Bai, J., Bernal-Llinares, M., Hewapathirana, S., Kundu, D. J., et al. (2019). The PRIDE database and related tools and resources in 2019: improving support for quantification data. Nucleic Acids Res. 47, D442–D450.
Presicce, P., Cappelletti, M., Senthamaraikannan, P., Ma, F., Morselli, M., Jackson, C. M., et al. (2020). TNF-signaling modulates neutrophil-mediated immunity at the feto-maternal interface during LPS-induced intrauterine inflammation. Front. Immunol. 11:558.
Rohlenova, K., Veys, K., Miranda-Santos, I., De Bock, K., and Carmeliet, P. (2018). Endothelial cell metabolism in health and disease. Trends Cell Biol. 28, 224–236. doi: 10.1016/j.tcb.2017.10.010
Shi, J., Zhao, Y., Wang, Y., Gao, W., Ding, J., Li, P., et al. (2014). Inflammatory caspases are innate immune receptors for intracellular LPS. Nature 514, 187–192. doi: 10.1038/nature13683
Xu, M., Wang, L., Wang, M., Wang, H., Zhang, H., Chen, Y., et al. (2019). Mitochondrial ROS and NLRP3 inflammasome in acute ozone-induced murine model of airway inflammation and bronchial hyperresponsiveness. Free Radic Res. 53, 780–790. doi: 10.1080/10715762.2019.1630735
Yang, X., Chang, Y., and Wei, W. (2016). Endothelial dysfunction and inflammation: immunity in rheumatoid arthritis. Mediators. Inflamm. 2016:6813016.
Yuan, H., Ma, J., Li, T., and Han, X. (2018). MiR-29b aggravates lipopolysaccharide-induced endothelial cells inflammatory damage by regulation of NF-kappaB and JNK signaling pathways. Biomed. Pharmacother. 99, 451–461. doi: 10.1016/j.biopha.2018.01.060
Keywords: endothelial-derived interleukin-1α, transendothelial neutrophils, lipopolysaccharide, Escherichia coli infection, iTRAQ
Citation: Liu X, Zhang H, He S, Mu X, Hu G and Dong H (2020) Endothelial-Derived Interleukin-1α Activates Innate Immunity by Promoting the Bactericidal Activity of Transendothelial Neutrophils. Front. Cell Dev. Biol. 8:590. doi: 10.3389/fcell.2020.00590
Received: 22 April 2020; Accepted: 17 June 2020;
Published: 07 July 2020.
Edited by:
Hao Sun, University of California, San Diego, United StatesReviewed by:
Lidija Radenovic, University of Belgrade, SerbiaTakashi Kato, Johns Hopkins University, United States
Copyright © 2020 Liu, Zhang, He, Mu, Hu and Dong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaoye Liu, xiaoyeliu@pku.edu.cn; Ge Hu, huge@bua.edu.cn; Hong Dong, donghongbua@163.com
 Xiaoye Liu
Xiaoye Liu Hui Zhang
Hui Zhang Shangwen He1
Shangwen He1