Abstract
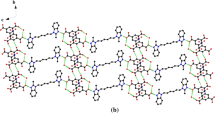
The results of growth from solutions of organic crystals of a new π-conjugated linear oligomer (Hex-O-Ph)2-BTD consisting of benzothiadiazole (BTD), phenyl (Ph) and oxazole (O) units and n-hexyl terminal substituents are presented. Well soluble at 20°C in n-hexane (1.7 g/L) and acetone (1.9 g/L), (Hex-O-Ph)2-BTD crystals are formed in the shape of films or plates up to 8 mm long and about 10 μm thick at the liquid–air interface within five days. The polymorphism and melting parameters of the new compound are determined by the method of differential scanning calorimetry. The structure of the single-crystal film is analyzed by Х-ray diffraction. The crystal structure of (Hex-O-Ph)2-BTD is found to be formed from close-packed (001) layers with a thickness of d001 = 2.39 nm.




Similar content being viewed by others
REFERENCES
R. A. Laudise, Ch. Kloc, P. G. Simpkins, and T. Siegrist, J. Cryst. Growth 187, 449 (1998). https://doi.org/10.1016/S0022-0248(98)00034-7
T. Yamao, T. Miki, H. Akagami, et al., Chem. Mater. 19, 3748 (2007). https://doi.org/10.1021/cm071051z
Y. Inada, T. Yamao, M. Inada, et al., Synth. Met. 161, 1869 (2011). https://doi.org/10.1016/j.synthmet.2011.06.026
V. A. Postnikov, Y. I. Odarchenko, A. V. Iovlev, et al., Cryst. Growth Des. 14, 1726 (2014). https://doi.org/10.1021/cg401876a
V. A. Postnikov, N. I. Sorokina, O. A. Alekseeva, et al., Crystallogr. Rep. 63 (1), 139 (2018). https://doi.org/10.1134/S0023476118050247
M. S. Skorotetcky, E. D. Krivtsova, O. V. Borshchev, et al., Dyes Pigm. 155, 284 (2018). https://doi.org/10.1016/j.dyepig.2018.03.043
V. A. Postnikov, N. I. Sorokina, A. A. Kulishov, et al., Acta Crystallogr., Sect. B: Struct. Sci., Cryst. Eng. Mater. 75, 1076 (2019). https://doi.org/10.1107/S2052520619012484
A. A. Kulishov, V. A. Postnikov, M. S. Lyasnikova, et al., Fiz. Tverd. Tela 61 (12), 2426 (2019). https://doi.org/10.21883/FTT.2019.12.48599.25ks
R. Hirase, M. Ishihara, T. Katagiri, et al., Org. Electron. 15, 1481 (2014). https://doi.org/10.1016/j.orgel.2014.04.010
M. S. Kazantsev, V. G. Konstantinov, D. I. Dominskiy, et al., Synth. Met. 232, 60 (2017). https://doi.org/10.1016/j.synthmet.2017.07.019
V. A. Postnikov, N. I. Sorokina, O. A. Alekseeva, et al., Crystallogr. Rep. 63 (5), 819 (2018). https://doi.org/10.1134/S1063774518050243
V. A. Postnikov, M. S. Lyasnikova, A. A. Kulishov, et al., Russ. J. Phys. Chem. A 93 (9), 1741 (2019). https://doi.org/10.1134/S0036024419090176
V. A. Postnikov, M. S. Lyasnikova, A. A. Kulishov, et al., Fiz. Tverd. Tela 61 (12), 2322 (2019). https://doi.org/10.21883/FTT.2019.12.48544.42ks
A. I. Kitaigorodskii, Molecular Crystals (Nauka, Moscow, 1971) [in Russian].
I. Vladimirov, M. Kellermeier, T. Geßner, et al., Nano Lett. 18, 9 (2018). https://doi.org/10.1021/acs.nanolett.7b03789
V. V. Bruevich, A. V. Glushkova, O. Yu. Poimanova, et al., ACS Appl. Mater. Interfaces 11, 6315 (2019). https://doi.org/10.1021/acsami.8b20700
Funding
This work was supported by the Russian Foundation for Basic Research (project no. 18-33-20050) and the Ministry of Education and Science of the Russian Federation (project no. RFMEF162119X0035); the equipment of the Centre for Collective Use of the Federal Research Center “Crystallography and Photonics”, Russian Academy of Sciences, and Centre for Collective Use “Centre of Polymer Investigation” of the Enikolopov Institute of Synthetic Polymer Materials, Russian Academy of Sciences.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Translated by P. Vlasov
Rights and permissions
About this article
Cite this article
Postnikov, V.A., Kulishov, A.A., Lyasnikova, M.S. et al. Crystals of Phenylene–Oxazole Oligomer with a Central Benzothiadiazole Fragment. J. Surf. Investig. 14, 540–543 (2020). https://doi.org/10.1134/S1027451020030386
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1027451020030386