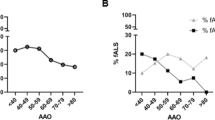
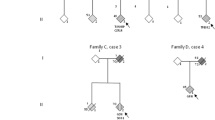
Abstract
Amyotrophic lateral sclerosis (ALS) is a neurodegenerative disease with clinical and etiological heterogeneity and a complex genetic contribution. Clinical, neuropathological, and genetic evidence revealed that ALS and frontotemporal dementia (FTD) are in part of a single disease continuum. Genetic causes have been identified in sporadic (SALS) and familial patients (FALS) and the recurrent genetic factor underlying ALS and FTD is the C9orf72 hexanucleotide repeat expansion (HRE). However, in our population, the concomitance of ALS and FTD cannot be explained by C9orf72 HRE in many FALS and SALS cases. Our aim is to further understand the genetic basis of ALS in Portuguese patients. 34 patients with FALS or SALS-FTD, negative for C9orf72 HRE, were screened for rare variants in a panel of 29 relevant genes by next-generation sequencing. We detected 15 variants in 11 genes, one classified as pathogenic in TARDBP, two as likely pathogenic in TARDBP and PRPH, and the others as variants of unknown significance (VUS). Gene variants, including VUS, were found in 41.2% FALS patients and 40% SALS-FTD. In most patients, no potential pathogenic variants were found. Our results emphasize the need to enhance the efforts to unravel the genetic architecture of ALS-FTD.

Similar content being viewed by others
Availability of data and material
Further data are available upon request.
References
van Es MA, Hardiman O, Chio A et al (2017) Amyotrophic lateral sclerosis. Lancet 390:2084–2098. https://doi.org/10.1016/S0140-6736(17)31287-4
Lomen-Hoerth C, Anderson T, Miller B (2002) The overlap of amyotrophic lateral sclerosis and frontotemporal dementia. Neurology 59:1077–1079. https://doi.org/10.1212/wnl.59.7.1077
Ryan M, Heverin M, Doherty MA et al (2018) Determining the incidence of familiality in ALS. Neurol Genet. https://doi.org/10.1212/NXG.0000000000000239
Byrne S, Heverin M, Elamin M et al (2013) Aggregation of neurologic and neuropsychiatric disease in amyotrophic lateral sclerosis kindreds: a population-based case-control cohort study of familial and sporadic amyotrophic lateral sclerosis. Ann Neurol 74:699–708. https://doi.org/10.1002/ana.23969
Gromicho M, Pinto S, Gisca E et al (2018) Frequency of C9orf72 hexanucleotide repeat expansion and SOD1 mutations in Portuguese patients with amyotrophic lateral sclerosis. Neurobiol Aging 70:325.e7–325.e15. https://doi.org/10.1016/j.neurobiolaging.2018.05.009
Zou Z-Y, Zhou Z-R, Che C-H et al (2017) Genetic epidemiology of amyotrophic lateral sclerosis: a systematic review and meta-analysis. J Neurol Neurosurg Psychiatry. https://doi.org/10.1136/jnnp-2016-315018
van der Zee J, Gijselinck I, Dillen L et al (2013) A Pan-European Study of the C9orf72 Repeat Associated with FTLD: Geographic Prevalence, Genomic Instability, and Intermediate Repeats. Hum Mutat 34:363–373. https://doi.org/10.1002/humu.22244
Cruts M, Gijselinck I, Van Langenhove T et al (2013) Current insights into the C9orf72 repeat expansion diseases of the FTLD/ALS spectrum. Trends Neurosci 36:450–459. https://doi.org/10.1016/j.tins.2013.04.010
Majounie E, Renton AE, Mok K et al (2012) Frequency of the C9orf72 hexanucleotide repeat expansion in patients with amyotrophic lateral sclerosis and frontotemporal dementia: a cross-sectional study. Lancet Neurol 11:323–330. https://doi.org/10.1016/S1474-4422(12)70043-1
Renton AE, Majounie E, Waite A et al (2011) A hexanucleotide repeat expansion in C9ORF72 is the cause of chromosome 9p21-linked ALS-FTD. Neuron 72:257–268. https://doi.org/10.1016/j.neuron.2011.09.010
DeJesus-Hernandez M, Mackenzie IR, Boeve BF et al (2011) Expanded GGGGCC hexanucleotide repeat in noncoding region of C9ORF72 causes chromosome 9p-linked FTD and ALS. Neuron 72:245–256. https://doi.org/10.1016/j.neuron.2011.09.011
Lattante S, Ciura S, Rouleau GA, Kabashi E (2015) Defining the genetic connection linking amyotrophic lateral sclerosis (ALS) with frontotemporal dementia (FTD). Trends Genet 31:263–273. https://doi.org/10.1016/j.tig.2015.03.005
Gao F, Almeida S, Lopez-Gonzalez R (2017) Dysregulated molecular pathways in amyotrophic lateral sclerosis–frontotemporal dementia spectrum disorder. EMBO J 36:2931–2950. https://doi.org/10.15252/embj.201797568
Ryan M, Heverin M, McLaughlin RL, Hardiman O (2019) Lifetime risk and heritability of amyotrophic lateral sclerosis. JAMA Neurol 76:1367. https://doi.org/10.1001/jamaneurol.2019.2044
Mathis S, Goizet C, Soulages A et al (2019) Genetics of amyotrophic lateral sclerosis: a review. J Neurol Sci 399:217–226. https://doi.org/10.1016/j.jns.2019.02.030
Chia R, Chiò A, Traynor BJ (2018) Novel genes associated with amyotrophic lateral sclerosis: diagnostic and clinical implications. Lancet Neurol 17:94–102. https://doi.org/10.1016/S1474-4422(17)30401-5
Farhan SMK, Howrigan DP, Abbott LE et al (2019) Exome sequencing in amyotrophic lateral sclerosis implicates a novel gene, DNAJC7, encoding a heat-shock protein. Nat Neurosci 22:1966–1974. https://doi.org/10.1038/s41593-019-0530-0
Nguyen HP, Van Broeckhoven C, van der Zee J (2018) ALS genes in the genomic era and their implications for FTD. Trends Genet 34:404–423. https://doi.org/10.1016/j.tig.2018.03.001
Al-Chalabi A, Calvo A, Chio A et al (2014) Analysis of amyotrophic lateral sclerosis as a multistep process: a population-based modelling study. Lancet Neurol 13:1108–1113. https://doi.org/10.1016/S1474-4422(14)70219-4
Vucic S, Higashihara M, Sobue G et al (2020) ALS is a multistep process in South Korean, Japanese, and Australian patients. Neurology. https://doi.org/10.1212/WNL.0000000000009015.10.1212/WNL.0000000000009015
Chiò A, Mazzini L, D’Alfonso S et al (2018) The multistep hypothesis of ALS revisited. Neurology 91:e635–e642. https://doi.org/10.1212/WNL.0000000000005996
Chiò A, Calvo A, Mazzini L et al (2012) Extensive genetics of ALS: a population-based study in Italy extensive genetics of ALS a population-based study in Italy. Neurology. https://doi.org/10.1212/WNL.0b013e3182735d36
Dols-Icardo O, García-Redondo A, Rojas-García R et al (2018) Analysis of known amyotrophic lateral sclerosis and frontotemporal dementia genes reveals a substantial genetic burden in patients manifesting both diseases not carrying the C9orf72 expansion mutation. J Neurol Neurosurg Psychiatry 89:162–168. https://doi.org/10.1136/jnnp-2017-316820
Turner MR, Al-Chalabi A, Chio A et al (2017) Genetic screening in sporadic ALS and FTD. J Neurol Neurosurg Psychiatry. https://doi.org/10.1136/jnnp-2017-315995
Brooks BR, Miller RG, Swash M, Munsat TL (2000) El Escorial revisited: Revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Mot Neuron Disord 1:293–299. https://doi.org/10.1080/146608200300079536
de Carvalho M, Dengler R, Eisen A et al (2008) Electrodiagnostic criteria for diagnosis of ALS. Clin Neurophysiol 119:497–503. https://doi.org/10.1016/j.clinph.2007.09.143
De Carvalho M, Ryczkowski A, Andersen P et al (2017) International survey of ALS experts about critical questions for assessing patients with ALS. Amyotroph Lateral Scler Front Degener. https://doi.org/10.1080/21678421.2017.1349150
Byrne S, Bede P, Elamin M et al (2011) Proposed criteria for familial amyotrophic lateral sclerosis. Amyotroph Lateral Scler 12:157–159. https://doi.org/10.3109/17482968.2010.545420
Rascovsky K, Hodges JR, Knopman D et al (2011) Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain 134:2456–2477. https://doi.org/10.1093/brain/awr179
Cedarbaum JM, Stambler N, Malta E et al (1999) The ALSFRS-R: a revised ALS functional rating scale that incorporates assessments of respiratory function. J Neurol Sci 169:13–21. https://doi.org/10.1016/S0022-510X(99)00210-5
Akimoto C, Volk AE, van Blitterswijk M et al (2014) A blinded international study on the reliability of genetic testing for GGGGCC-repeat expansions in C9orf72 reveals marked differences in results among 14 laboratories. J Med Genet 51:419–424. https://doi.org/10.1136/jmedgenet-2014-102360
McKenna A, Hanna M, Banks E et al (2010) The Genome Analysis Toolkit: a MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res 20:1297–1303. https://doi.org/10.1101/gr.107524.110
Garrison E, Marth G (2012) Haplotype-based variant detection from short-read sequencing. arXiv pre-print Serv
Li H, Handsaker B, Wysoker A et al (2009) The sequence alignment/map format and SAMtools. Bioinformatics 25:2078–2079. https://doi.org/10.1093/bioinformatics/btp352
Whiffin N, Minikel E, Walsh R et al (2017) Using high-resolution variant frequencies to empower clinical genome interpretation. Genet Med 19:1151–1158. https://doi.org/10.1038/gim.2017.26
van der Spek RAA, van Rheenen W, Pulit SL et al (2019) The project MinE databrowser: bringing large-scale whole-genome sequencing in ALS to researchers and the public. Amyotroph Lateral Scler Front Degener 20:432–440. https://doi.org/10.1080/21678421.2019.1606244
Cirulli ET, Lasseigne BN, Petrovski S et al (2015) Exome sequencing in amyotrophic lateral sclerosis identifies risk genes and pathways. Science 347:1436–1441. https://doi.org/10.1126/science.aaa3650
Richards S, Aziz N, Bale S et al (2015) Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med 17:405–424. https://doi.org/10.1038/gim.2015.30
Schwarz JM, Cooper DN, Schuelke M, Seelow D (2014) MutationTaster2: mutation prediction for the deep-sequencing age. Nat Methods 11:361–362. https://doi.org/10.1038/nmeth.2890
Adzhubei IA, Schmidt S, Peshkin L et al (2010) A method and server for predicting damaging missense mutations. Nat Methods 7:248
Sim N-L, Kumar P, Hu J et al (2012) SIFT web server: predicting effects of amino acid substitutions on proteins. Nucleic Acids Res 40:W452–W457
Ioannidis NM, Rothstein JH, Pejaver V et al (2016) REVEL: an ensemble method for predicting the pathogenicity of rare missense variants. Am J Hum Genet 99:877–885. https://doi.org/10.1016/j.ajhg.2016.08.016
Reva B, Antipin Y, Sander C (2007) Determinants of protein function revealed by combinatorial entropy optimization. Genome Biol 8:R232. https://doi.org/10.1186/gb-2007-8-11-r232
Dong C, Wei P, Jian X et al (2015) Comparison and integration of deleteriousness prediction methods for nonsynonymous SNVs in whole exome sequencing studies. Hum Mol Genet 24:2125–2137. https://doi.org/10.1093/hmg/ddu733
Davydov EV, Goode DL, Sirota M et al (2010) Identifying a high fraction of the human genome to be under selective constraint using GERP++. PLOS Comput Biol 6:e1001025
McLaren W, Gil L, Hunt SE et al (2016) The ensembl variant effect predictor. Genome Biol 17:122. https://doi.org/10.1186/s13059-016-0974-4
Liu X, Wu C, Li C, Boerwinkle E (2016) dbNSFP v3.0: a one-stop database of functional predictions and annotations for human nonsynonymous and splice-site SNVs. Hum Mutat 37:235–241. https://doi.org/10.1002/humu.22932
Fadista J, Oskolkov N, Hansson O, Groop L (2017) LoFtool: a gene intolerance score based on loss-of-function variants in 60 706 individuals. Bioinformatics 33:471–474. https://doi.org/10.1093/bioinformatics/btv602
Jian X, Boerwinkle E, Liu X (2014) In silico prediction of splice-altering single nucleotide variants in the human genome. Nucleic Acids Res 42:13534–13544. https://doi.org/10.1093/nar/gku1206
Lattante S, Rouleau GA, Kabashi E (2013) TARDBP and FUS mutations associated with amyotrophic lateral sclerosis: summary and update. Hum Mutat 34:812–826. https://doi.org/10.1002/humu.22319
Corrado L, Carlomagno Y, Falasco L et al (2011) A novel peripherin gene (PRPH) mutation identified in one sporadic amyotrophic lateral sclerosis patient. Neurobiol Aging 32:552.e1–552.e6. https://doi.org/10.1016/j.neurobiolaging.2010.02.011
Leung CL, He CZ, Kaufmann P et al (2004) A pathogenic peripherin gene mutation in a patient with amyotrophic lateral sclerosis. Brain Pathol 14:290–296. https://doi.org/10.1111/j.1750-3639.2004.tb00066.x
Cady J, Allred P, Bali T et al (2015) Amyotrophic lateral sclerosis onset is influenced by the burden of rare variants in known amyotrophic lateral sclerosis genes. Ann Neurol 77:100–113. https://doi.org/10.1002/ana.24306
Millecamps S, Salachas F, Cazeneuve C et al (2010) SOD1, ANG, VAPB, TARDBP, and FUS mutations in familial amyotrophic lateral sclerosis: Genotype-phenotype correlations. J Med Genet 47:554–560. https://doi.org/10.1136/jmg.2010.077180
Project MinE ALS Sequencing Consortium (2018) Project MinE: study design and pilot analyses of a large-scale whole-genome sequencing study in amyotrophic lateral sclerosis. Eur J Hum Genet 26:1537–1546. https://doi.org/10.1038/s41431-018-0177-4
Pensato, Magri, Bella et al (2020) Sorting rare ALS genetic variants by targeted re-sequencing panel in Italian patients: OPTN, VCP, and SQSTM1 variants account for 3% of rare genetic forms. J Clin Med 9:412. https://doi.org/10.3390/jcm9020412
Tripolszki K, Gampawar P, Schmidt H et al (2019) Comprehensive genetic analysis of a hungarian amyotrophic lateral sclerosis cohort. Front Genet 10:732. https://doi.org/10.3389/fgene.2019.00732
Müller K, Brenner D, Weydt P et al (2018) Comprehensive analysis of the mutation spectrum in 301 German ALS families. J Neurol Neurosurg Psychiatry 89:817–827. https://doi.org/10.1136/jnnp-2017-317611
Kenna KP, McLaughlin RL, Byrne S et al (2013) Delineating the genetic heterogeneity of ALS using targeted high-throughput sequencing. J Med Genet 50:776–783. https://doi.org/10.1136/jmedgenet-2013-101795
Kabashi E, Valdmanis PN, Dion P et al (2008) TARDBP mutations in individuals with sporadic and familial amyotrophic lateral sclerosis. Nat Genet 40:572–574. https://doi.org/10.1038/ng.132
Orrù S, Manolakos E, Orrù N et al (2012) High frequency of the TARDBP p.Ala382Thr mutation in Sardinian patients with amyotrophic lateral sclerosis. Clin Genet 81:172–178. https://doi.org/10.1111/j.1399-0004.2011.01668.x
Borghero G, Pugliatti M, Marrosu F et al (2014) Genetic architecture of ALS in Sardinia. Neurobiol Aging 35:2882.e7–2882.e12. https://doi.org/10.1016/j.neurobiolaging.2014.07.012
Gros-Louis F, Larivière R, Gowing G et al (2004) A frameshift deletion in peripherin gene associated with amyotrophic lateral sclerosis. J Biol Chem 279:45951–45956. https://doi.org/10.1074/jbc.M408139200
Orlacchio A, Babalini C, Borreca A et al (2010) SPATACSIN mutations cause autosomal recessive juvenile amyotrophic lateral sclerosis. Brain 133:591–598. https://doi.org/10.1093/brain/awp325
Couthouis J, Raphael AR, Daneshjou R, Gitler AD (2014) Targeted exon capture and sequencing in sporadic amyotrophic lateral sclerosis. PLoS Genet 10:e1004704. https://doi.org/10.1371/journal.pgen.1004704
Nakamura R, Sone J, Atsuta N et al (2016) Next-generation sequencing of 28 ALS-related genes in a Japanese ALS cohort. Neurobiol Aging 39:219.e1–219.e8. https://doi.org/10.1016/j.neurobiolaging.2015.11.030
Nishiyama A, Niihori T, Warita H et al (2017) Comprehensive targeted next-generation sequencing in Japanese familial amyotrophic lateral sclerosis. Neurobiol Aging 53:194.e1–194.e8. https://doi.org/10.1016/j.neurobiolaging.2017.01.004
Parkinson N, Ince PG, Smith MO et al (2006) ALS phenotypes with mutations in CHMP2B (charged multivesicular body protein 2B). Neurology 67:1074–1077. https://doi.org/10.1212/01.wnl.0000231510.89311.8b
Cox LE, Ferraiuolo L, Goodall EF et al (2010) Mutations in CHMP2B in lower motor neuron predominant amyotrophic lateral sclerosis (ALS). PLoS ONE 5:e9872. https://doi.org/10.1371/journal.pone.0009872
van Blitterswijk M, Vlam L, van Es MA et al (2012) Genetic overlap between apparently sporadic motor neuron diseases. PLoS ONE 7:e48983. https://doi.org/10.1371/journal.pone.0048983
Narain P, Pandey A, Gupta S et al (2018) Targeted next-generation sequencing reveals novel and rare variants in Indian patients with amyotrophic lateral sclerosis. Neurobiol Aging 71:265.e9–265.e14. https://doi.org/10.1016/j.neurobiolaging.2018.05.012
Hermosura MC, Nayakanti H, Dorovkov MV et al (2005) A TRPM7 variant shows altered sensitivity to magnesium that may contribute to the pathogenesis of two Guamanian neurodegenerative disorders. Proc Natl Acad Sci USA 102:11510–11515. https://doi.org/10.1073/pnas.0505149102
Sun Y, Sukumaran P, Schaar A, Singh BB (2015) TRPM7 and its role in neurodegenerative diseases. Channels 9:253–261. https://doi.org/10.1080/19336950.2015.1075675
Zhou S-L, Tan C-C, Hou X-H et al (2019) TREM2 variants and neurodegenerative diseases: a systematic review and meta-analysis. J Alzheimers Dis 68:1171–1184. https://doi.org/10.3233/JAD-181038
Su W-H, Shi Z-H, Liu S-L et al (2018) The rs75932628 and rs2234253 polymorphisms of the TREM2 gene were associated with susceptibility to frontotemporal lobar degeneration in Caucasian populations. Ann Hum Genet 82:177–185. https://doi.org/10.1111/ahg.12241
Cady J, Koval ED, Benitez BA et al (2014) TREM2 variant p. R47H as a risk factor for sporadic amyotrophic lateral sclerosis. JAMA Neurol 71:449–453. https://doi.org/10.1001/jamaneurol.2013.6237
Ogaki K, Heckman MG, Koga S et al (2018) Association study between multiple system atrophy and TREM2 p. R47H. Neurol Genet 4:e257–e257. https://doi.org/10.1212/NXG.0000000000000257
Jin SC, Benitez BA, Karch CM et al (2014) Coding variants in TREM2 increase risk for Alzheimer’s disease. Hum Mol Genet 23:5838–5846. https://doi.org/10.1093/hmg/ddu277
Giannoccaro MP, Bartoletti-Stella A, Piras S et al (2017) Multiple variants in families with amyotrophic lateral sclerosis and frontotemporal dementia related to C9orf72 repeat expansion: further observations on their oligogenic nature. J Neurol 264:1426–1433. https://doi.org/10.1007/s00415-017-8540-x
Kwiatkowski TJ, Bosco DA, Leclerc AL et al (2009) Mutations in the FUS/TLS gene on chromosome 16 cause familial amyotrophic lateral sclerosis. Science 323:1205–1208. https://doi.org/10.1126/science.1166066
Vance C, Rogelj B, Hortobagyi T et al (2009) Mutations in FUS, an RNA processing protein, cause familial amyotrophic lateral sclerosis type 6. Science 323:1208–1211. https://doi.org/10.1126/science.1165942
Mejzini R, Flynn LL, Pitout IL et al (2019) ALS genetics, mechanisms, and therapeutics: where are we now? Front Neurosci 13:1–27. https://doi.org/10.3389/fnins.2019.01310
Naumann M, Peikert K, Günther R et al (2019) Phenotypes and malignancy risk of different FUS mutations in genetic amyotrophic lateral sclerosis. Ann Clin Transl Neurol 6:2384–2394. https://doi.org/10.1002/acn3.50930
Iko Y, Kodama TS, Kasai N et al (2004) Domain architectures and characterization of an RNA-binding protein, TLS. J Biol Chem 279:44834–44840. https://doi.org/10.1074/jbc.M408552200
Shang Y, Huang EJ (2016) Mechanisms of FUS mutations in familial amyotrophic lateral sclerosis. Brain Res 1647:65–78. https://doi.org/10.1016/j.brainres.2016.03.036
Merner ND, Girard SL, Catoire H et al (2012) Exome sequencing identifies FUS mutations as a cause of essential tremor. Am J Hum Genet 91:313–319. https://doi.org/10.1016/j.ajhg.2012.07.002
Yan J, Deng HX, Siddique N et al (2010) Frameshift and novel mutations in FUS in familial amyotrophic lateral sclerosis and ALS/dementia. Neurology 75:807–814. https://doi.org/10.1212/WNL.0b013e3181f07e0c
Akiyama T, Warita H, Kato M et al (2016) Genotype–phenotype relationships in familial amyotrophic lateral sclerosis with FUS/TLS mutations in Japan. Muscle Nerve 54:398–404. https://doi.org/10.1002/mus.25061
Van Langenhove T, van der Zee J, Sleegers K et al (2010) Genetic contribution of FUS to frontotemporal lobar degeneration. Neurology 74:366–371. https://doi.org/10.1212/WNL.0b013e3181ccc732
Freischmidt A, Wieland T, Richter B et al (2015) Haploinsufficiency of TBK1 causes familial ALS and fronto-temporal dementia. Nat Neurosci 18:631–636. https://doi.org/10.1038/nn.4000
Costa MR, Gromicho M, Pronto-Laborinho AC et al (2019) Novel TBK1 LoF variant in a family with upper motor neuron predominant motor neuron disease. J Neurol Sci 403:117–118. https://doi.org/10.1016/j.jns.2019.06.029
van der Zee J, Gijselinck I, Van Mossevelde S et al (2017) TBK1 mutation spectrum in an extended European patient cohort with frontotemporal dementia and amyotrophic lateral sclerosis. Hum Mutat 38:297–309. https://doi.org/10.1002/humu.23161
Freischmidt A, Müller K, Ludolph AC et al (2017) Association of mutations in TBK1 with sporadic and familial amyotrophic lateral sclerosis and frontotemporal dementia. JAMA Neurol 74:110–113. https://doi.org/10.1001/jamaneurol.2016.3712
Cui R, Tuo M, Li P, Zhou C (2018) Association between TBK1 mutations and risk of amyotrophic lateral sclerosis/frontotemporal dementia spectrum: a meta-analysis. Neurol Sci 39:811–820. https://doi.org/10.1007/s10072-018-3246-0
Williams KL, Topp S, Yang S et al (2016) CCNF mutations in amyotrophic lateral sclerosis and frontotemporal dementia. Nat Commun 7:11253. https://doi.org/10.1038/ncomms11253
Takahashi Y, Fukuda Y, Yoshimura J et al (2013) ERBB4 mutations that disrupt the neuregulin-ErbB4 pathway cause amyotrophic lateral sclerosis type 19. Am J Hum Genet 93:900–905. https://doi.org/10.1016/j.ajhg.2013.09.008
Sun L, Cheng B, Zhou Y et al (2020) ErbB4 mutation that decreased NRG1-ErbB4 signaling involved in the pathogenesis of amyotrophic lateral sclerosis/frontotemporal dementia. J Alzheimer’s Dis. https://doi.org/10.3233/JAD-191230
Van Blitterswijk M, Van Es MA, Hennekam EAM et al (2012) Evidence for an oligogenic basis of amyotrophic lateral sclerosis. Hum Mol Genet 21:3776–3784. https://doi.org/10.1093/hmg/dds199
Lattante S, Doronzio PN, Marangi G et al (2019) Coexistence of variants in TBK1 and in other ALS-related genes elucidates an oligogenic model of pathogenesis in sporadic ALS. Neurobiol Aging 84:239.e9–239.e14. https://doi.org/10.1016/j.neurobiolaging.2019.03.010
Zhang H, Cai W, Chen S et al (2018) Screening for possible oligogenic pathogenesis in Chinese sporadic ALS patients. Amyotroph Lateral Scler Frontotemporal Degener 19:419–425. https://doi.org/10.1080/21678421.2018.1432659
Funding
This project was supported by FTC—Fundação para a Ciência e Tecnologia—through project “Comprehensive evaluation of circulating MicroRNA as diagnostic and prognostic biomarkers in Amyotrophic Lateral Sclerosis”—PTDC/MEC-NEU/31195/2017.
Author information
Authors and Affiliations
Contributions
Conceptualization, MG and MdC; methodology, ACPL and JT; software, MG and JT; validation, DA; formal analysis, MG, AMC, and RR; data curation, MG, AMC, RR, and DA; writing—original draft preparation, MG; writing—review and editing, MG and MdC; supervision, MdC.
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflict of interest.
Ethics approval
This project was approved by the Local Ethics Committee (ID number 215/15). The study conformed to the standards defined in the latest revision of the Declaration of Helsinki. All patients and controls signed a written informed consent. Databases were properly treated for privacy.
Consent to participate
All listed authors have approved the manuscript before submission, including the names and order of authors.
Consent for publication
All listed authors have approved the version to be published.
Rights and permissions
About this article
Cite this article
Gromicho, M., Coutinho, A.M., Pronto-Laborinho, A.C. et al. Targeted next-generation sequencing study in familial ALS-FTD Portuguese patients negative for C9orf72 HRE. J Neurol 267, 3578–3592 (2020). https://doi.org/10.1007/s00415-020-10042-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-020-10042-y