Abstract
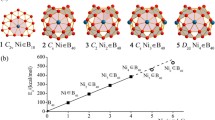
B-C binary monolayers and fullerenes (borafullerenes) have received considerable attention in recent years. Inspired by the newly reported B4C3 semiconducting boron carbide monolayer isovalent to graphene (Tian et al., Nanoscale, 2019, 11, 11099), we predict herein at density functional theory level a new class of borafullerenes (1–8) following the isolated B4C3 hexagonal pyramid rule. The spherically aromatic borafullerenes C5h B20C35 (1), C5 B20C45 (2), C5h B20C55 (3), and C5 B20C65 (4) isovalent to C50, C60, C70, and C80, respectively, possess five isolated B4C3 hexagonal pyramids evenly distributed on the waist around the C5 molecular axis, while S10 B40C50 (5), C5 B40C60 (6), S10 B40C70 (7), and C5 B40C80 (8) encompass ten isolated B4C3 pyramids symmetrically distributed on the cage surface. Detailed orbital and bonding analyses indicate that these borafullerenes follow similar σ and π-bonding patterns with their fullerene analogues, with three delocalized 7c-2e π bonds forming a local π-aromatic system over each isolated B4C3 hexagonal pyramid. The calculated formation energies of the (B4C3)nC60-6n (n = 1–5) series isovalent to C60 appear to increase almost linearly with the number of isolated B4C3 pyramids in the system. The IR, Raman, and UV-vis spectra of the prototypical B20C45 (2) are theoretically simulated to facilitate its future spectral characterization.




Similar content being viewed by others
References
Kroto HW (1987) The stability of the fullerenes Cn, with n = 24, 28, 32, 36, 50, 60 and 70. Nature (London, United Kingdom) 329:529–531. https://doi.org/10.1038/329529a0
Schmalz TG, Seitz WA, Klein DJ, Hite GE (1988) Elemental carbon cages. J. Am. Chem. Soc. 110:1113–1127. https://doi.org/10.1021/ja00212a020
Kroto HW, Heath JR, O’Brien SC, Curl RF, Smalley RE (1985) C60: buckminsterfullerene. Nature 318:162–163. https://doi.org/10.1038/318162a0
Lamparth I, Nuber B, Schick G, Skiebe A, Grӧsser T, Hirsch A (1995) C59N+ and C69N+: isoelectronic heteroanalogues of C60 and C70. Angew. Chem. 107: 2473-2476. https://doi.org/10.1002/ange.19951072035; Angew. Chem. Int. Ed. Engl. 34: 2257–2259. https://doi.org/10.1002/anie.199522571
Nuber B, Hirsch A (1996) A new route to nitrogen heterofullerenes and the first synthesis of (C69N)2. Chem. Commun. 12:1421–1422. https://doi.org/10.1039/cc9960001421
Guo T, Jin CM, Smalley RE (1991) Doping bucky: formation and properties of boron-doped buckminsterfullerene. J. Phys. Chem. 95:4948–4950. https://doi.org/10.1021/j100166a010
Kimura T, Sugai T, Shinohara H (1996) Production and characterization of boron- and silicon-doped carbon clusters. Chem. Phys. Lett. 256:269–273. https://doi.org/10.1016/0009-2614(96)00436-8
Muhr HJ, Nesper R, Schnyder B, Kötz R (1996) The boron heterofullerenes C59B and C69B: generation, extraction, mass spectrometric and XPS characterization. Chem. Phys. Lett. 249:399–405. https://doi.org/10.1016/0009-2614(95)01451-9
Dunk PW, Rodrίguez-Fortea A, Kaiser NK, Shinohara H, Poblet JM, Kroto HW (2013) Formation of heterofullerenes by direct exposure of C60 to boron vapor. Angew. Chem. Int. Ed. 52:315–319. https://doi.org/10.1002/anie.201208244
Kurita N, Kobayashi K, Kumahora H, Tago K, Ozawa K (1992) Molecular structures, binding energies and electronic properties of dopyballs C59X (X= B, N and S). Chem. Phys. Lett. 198:95–99. https://doi.org/10.1016/0009-2614(92)90054-Q
Kurita N, Kobayashi K, Kumahora H, Tago K (1993) Bonding and electronic properties of substituted fullerenes C58B2 and C58N2. Phys. Rev. B 48:4850–4854. https://doi.org/10.1080/15363839308011901
Miyamoto Y, Hamada N, Oshiyama A, Saito S (1992) Electronic structures of solid BC59. Phys. Rev. B 46:1749–1753. https://doi.org/10.1103/PhysRevB.46.1749
Zou Y, Luo MX, Wang ZJ, Li WZ (1998) Properties of boron-substituted heterofullerenes. Chin. Phys. Lett. 15:106–108. https://doi.org/10.1088/0256-307X/15/2/011
Chen ZF, Zhao XZ, Tang A (1999) Theoretical studies of the substitution patterns in heterofullerenes C60-xNx and C60-xBx (x=2-8). J. Phys. Chem. A 103:10961–10968. https://doi.org/10.1021/jp9908707
Xie RH, Jensen L, Bryant GW, Zhao JJ, Smith Jr VH (2003) Structural, electronic, and magnetic properties of heterofullerene C48B12. Chem. Phys. Lett. 375:445–451. https://doi.org/10.1016/S0009-2614(03)00879-0
Garg I, Sharma H, Dharamvir K, Jindal VK (2011) Substitutional patterns in boron doped heterofullerenes C60−nBn (n = 1–12). J. Comput. Theor. Nanosci. 8:642–655. https://doi.org/10.1166/jctn.2011.1734
Mohr S, Pochet P, Amsler M, Schaefer B, Sadeghi A, Genovese L, Goedecker S (2014) Boron aggregation in the ground states of boron-carbon fullerenes. Phys. Rev. B 89:041404(R). https://doi.org/10.1103/PhysRevB.89.041404
Yan M, Tian XX, Pei L, Li SD (2018) Cage-like B40C30, B40C40, and B40C50: high-symmetry heterofullerenes isovalent with C60, C70, and C80. J. Mol. Model. 24:296. https://doi.org/10.1007/s00894-018-3828-z
Tian XX, Xuan XY, Yu M, Mu YW, Lu HG, Zhang ZH, Li SD (2019) Predicting two-dimensional semiconducting boron carbides. Nanoscale 11:11099–11106. https://doi.org/10.1039/c9nr02681a
Chen X, Zhao YF, Wang LS, Li J (2017) Recent progresses of global minimum searches of nanoclusters with a constrained basin-hopping algorithm in the TGMin program. Comput. Theor. Chem. 1107:57–65. https://doi.org/10.1016/j.comptc.2016.12.028
Zhao YF, Chen X, Li J (2017) TGMin: a global-minimum structure search program based on a constrained basin-hopping algorithm. Nano. Res. 10:3407–3420. https://doi.org/10.1007/s12274-017-1553-z
Chen X, Zhao YF, Zhang YY, Li J (2019) TGMin: an efficient global minimum searching program for free and surface-supported clusters. J. Comput. Chem. 40:1105–1112. https://doi.org/10.1002/jcc.25649
Goedecker S, Hellmann W, Lenosky T (2005) Global minimum determination of the Born-Oppenheimer surface within density functional theory. Phys. Rev. Lett. 95:055501. https://doi.org/10.1103/PhysRevLett.95.055501
Goedecker S (2004) Minima hopping: an efficient search method for the global minimum of the potential energy surface of complex molecular systems. J. Chem. Phys. 120:9911–9917. https://doi.org/10.1063/1.1724816
Adamo C, Barone V (1999) Toward reliable density functional methods without adjustable parameters: the PBE0 model. J. Chem. Phys. 110:6158–6170. https://doi.org/10.1063/1.478522
Krishnan R, Binkley JS, Seeger R, Pople JA (1980) Self-consistent molecular orbital methods. XX. A basis set for correlated wave functions. J. Chem. Phys. 72:650–654. https://doi.org/10.1063/1.438955
Zhai HJ, Zhao YF, Li WL, Chen Q, Bai H, Hu HS, Piazza ZA, Tian WJ, Lu HG, Wu YB, Mu YW, Wei GF, Liu ZP, Li J, Li SD, Wang LS (2014) Observation of an all-boron fullerene. Nat. Chem. 6:727–731. https://doi.org/10.1038/nchem.1999
Chen Q, Li WL, Zhao YF, Zhang SY, Hu HS, Bai H, Li HR, Tian WJ, Lu HG, Zhai HJ, Li SD, Li J, Wang LS (2015) Experimental and theoretical evidence of an axially chiral borospherene. ACS. Nano. 9:754–760. https://doi.org/10.1021/nn506262c
Chen Q, Zhang SY, Bai H, Tian WJ, Gao T, Li HR, Miao CQ, Mu YW, Lu HG, Zhai HJ, Li SD (2015) Cage-like B41+ and B422+: new chiral members of the borospherene family. Angew. Chem. Int. Ed. 54:8160–8164. https://doi.org/10.1002/anie.201501588
Wang LS (2016) Photoelectron spectroscopy of size-selected boron clusters: from planar structures to borophenes and borospherenes. Int. Rev. Phys. Chem. 35:69–142. https://doi.org/10.1080/0144235X.2016.1147816
Jian T, Chen XN, Li SD, Boldyrev AI, Li J, Wang LS (2019) Probing the structures and bonding of size-selected boron and doped-boron clusters. Chem. Soc. Rev. 48:3550–3591. https://doi.org/10.1039/c9cs00233b
Zubarev DY, Boldyrev AI (2008) Developing paradigms of chemical bonding: adaptive natural density partitioning. Phys. Chem. Chem. Phys. 10:5207–5217. https://doi.org/10.1039/b804083d
Zubarev DY, Boldyrev AI (2008) Revealing intuitively assessable chemical bonding patterns in organic aromatic molecules via adaptive natural density partitioning. J. Org. Chem. 73:9251–9258. https://doi.org/10.1021/jo801407e
Tkachenko NV, Boldyrev AI (2019) Chemical bonding analysis of excited states using the adaptive natural density partitioning method. Phys. Chem. Chem. Phys. 21:9590–9596. https://doi.org/10.1039/c9cp00379g
Schleyer PvR, Maerker C, Dransfeld A, Jiao HJ, van Eikema Hommes NJR (1996) Nucleus-independent chemical shifts: a simple and efficient aromaticity probe. J. Am. Chem. Soc. 118:6317–6318. https://doi.org/10.1021/JA960582D
Chen ZF, Wannere CS, Corminboeuf C, Puchta R, Schleyer PvR (2005) Nucleus-independent chemical shifts (NICS) as an aromaticity criterion. Chem. Rev. 105:3842–3888. https://doi.org/10.1021/cr030088+
Bauernschmitt R, Ahlrichs R (1996) Treatment of electronic excitations within the adiabatic approximation of time dependent density functional theory. Chem. Phys. Lett. 256:454–464. https://doi.org/10.1016/0009-2614(96)00440-X
Casida ME, Jamorski C, Casida KC, Salahub DR (1998) Molecular excitation energies to high-lying bound states from time-dependent density-functional response theory: characterization and correction of the time-dependent local density approximation ionization threshold. J. Chem. Phys. 108:4439–4449. https://doi.org/10.1063/1.475855
VandeVondele J, Krack M, Mohamed F, Parrinello M, Chassaing T, Hutter J (2005) QUICKSTEP: fast and accurate density functional calculations using a mixed Gaussian and plane waves approach. Comput. Phys. Commun. 167:103–128. https://doi.org/10.1016/j.cpc.2004.12.014
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA et al (2009) Gaussian 09, revision D.01. Gaussian, Inc., Wallingford
Wang GJ, Zhou MF, Goettel JT, Schrobilgen GJ, Su J, Li J, Schlöder T, Riedel S (2014) Identification of an iridium-containing compound with a formal oxidation state of IX. Nature 514:475–477. https://doi.org/10.1038/nature13795
Fagiani MR, Song XW, Petkov P, Debnath S, Gewinner S, Schöllkopf W, Heine T (2017) Untersuchung der struktur und dynamik des B13+ mithilfe der Infrarot-p hotodissoziationsspektroskopie. Angew. Chem. 129:515–519. https://doi.org/10.1002/ange.201609766
Ciuparu D, Klie RF, Zhu YM, Pfefferle L (2004) Synthesis of pure boron single-wall nanotubes. J. Phys. Chem. B 108:3967–3969. https://doi.org/10.1021/jp049301b
Funding
This work was supported by the National Natural Science Foundation of China (21720102006 and 21973057 to S.-D. Li, 21903049 to X.-X. Tian, 21803037 to W.-Y. Zan and 11504213 to Y.-W. Mu).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 4.82;mb)
Rights and permissions
About this article
Cite this article
Yan, M., Tian, XX., Pei, L. et al. Novel B-C binary fullerenes following the isolated B4C3 hexagonal pyramid rule. J Mol Model 26, 199 (2020). https://doi.org/10.1007/s00894-020-04425-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-020-04425-1