Abstract
5-(Diethylammoniothio)-1,3-dimethylbarbituric acid (8) was obtained in good yield from the reaction of 5-bromo-1,3-dimethylbarbituric acid (5) and 1,3-diethylthiourea. The obtained product has been characterized using different techniques including single-crystal X-ray diffraction, FTIR, MS and NMR spectroscopy. For this reaction, a detailed computational study of the reaction steps has been performed using density functional theory (DFT). Both thermodynamic and kinetic parameters were calculated. Step B is the most favorable reaction with an activation energy of 33 kJ mol−1 using the solvation model that based on the solvent model density (SMD) calculated at B3LYP/6-31G(d).
Graphic abstract
The rate-determining step of the most plausible mechanism involves the formation of C-S bond via proton transfer to oxygen (intramolecular attraction) with an activation barrier of 33 kJ mol-1 using the solvation model that based on density (SMD) solvent model calculated at B3LYP/6-31G(d).










Similar content being viewed by others
References
Barakat A, Islam MS, Al-Majid AM, Soliman SM, Mabkhot YN, Al-Othman ZA, Ghabbour HA, Fun H-K (2015) Synthesis of novel 5-monoalkylbarbiturate derivatives: new access to 1, 2-oxazepines. Tetrahedron Lett 56(50):6984–6987
Sharma A, Jad Y, Siddiqui MR, Torre BG, Albericio F, El-Faham A (2017) Synthesis, characterization, and tautomerism of 1, 3-dimethyl pyrimidine-2, 4, 6-trione s-triazinyl hydrazine/hydrazone derivatives. J Chem 2017
Sweidan K, Abu-Salem Q, Al-Sheikh A, Sheikha GA (2009) Novel derivatives of 1, 3-dimethyl-5-methylenebarbituric acid. Lett Org Chem 6(8):669–672
Mallah E, Sweidan K, Engelmann J, Steimann M, Kuhn N, Maier ME (2012) Nucleophilic substitution approach towards 1, 3-dimethylbarbituric acid derivatives—new synthetic routes and crystal structures. Tetrahedron 68(4):1005–1010
Al-Sheikh A, Sweidan K, Sweileh B, Steimann M, Schubert H, Kuhn N (2008) Synthesis and crystal structure of triethylammonium 5-[(2, 2-dimethyl-4, 6-dioxo-1, 3-dioxan-5-ylidene)(methylthio) methyl]-1, 3-dimethylpyrimidine-2, 4, 6-trionate. Z Naturforsch B 63(8):1020–1022
Sweidan K, Al-Sheikh A, Sweileh B, Sunjuk M, Kuhn N (2009) Synthesis of phosphorus, arsenic and antimony ylides containing the 1, 3-dimethyl-2, 4, 6 (1H, 3H, 5H)-pyrimidinetrione fragments. Lett Org Chem 6(1):1–3
Zade MN, Katiya MM, Sontakke MM, Dhonde MG, Berad BN, Thakare VJ, Bhaskar CS (2018) CS and CN coupling reactions of barbituric acid via selective and complete bromination using greener KBr/H2O2 as a brominating agent. J Indian Chem Soc 95(5):553–558
Franssen M, Van der Plas H (1987) The chlorination of barbituric acid and some of its derivatives by chloroperoxidase. Bioorg Chem 15(1):59–70
Sweidan K, Rayyan WA, Zarga MA, El-Abadelah M, Mohammad AY (2014) Synthesis and antibacterial evaluation of model fluoroquinolone-benzylidene barbiturate hybrids. Lett Org Chem 11(6):422–425
Sweidan K, El-Abadelah MM, Haddad SF, Voelter W (2015) Synthesis and characterization of some new fluoroquinolone-barbiturate hybrid systems. Z Naturforschung B 70(7):513–517
Chakravarthy RD, Ramkumar V, Chand DK (2014) A molybdenum based metallomicellar catalyst for controlled and selective sulfoxidation reactions in aqueous medium. Green Chem 16(4):2190–2196
Sipos G, Drinkel EE, Dorta R (2015) The emergence of sulfoxides as efficient ligands in transition metal catalysis. Chem Soc Rev 44(11):3834–3860
Yu X, Liu Y, Li Y, Wang Q (2016) Design, synthesis, acaricidal/insecticidal activity, and structure-activity relationship studies of novel oxazolines containing sulfone/sulfoxide groups based on the sulfonylurea receptor protein-binding site. J Agric Food Chem 64(15):3034–3040
Sheldrick GM (2008) A short history of SHELX. Acta Crystallogr A 64(1):112–122
Frisch M, Trucks G, Schlegel H, Scuseria G, Robb M, Cheeseman J, Scalmani G, Barone V, Petersson G, Nakatsuji H (2016) Gaussian 16, revision a.03. Gaussian, Inc., Wallingford, CT
Sweidan K, AlDamen MA, Maichle-Mößmer C, Mubarak MS (2012) Synthesis, crystal structure and thermodynamic calculations of 1, 3-diethyl-5-(diethylaminium) methylene-2-thiobarbituric acid adduct. J Chem Crystallogr 42(5):427–431
Ralhan S, Ray N (2003) Density functional study of barbituric acid and its tautomers. J Mol Struct Theochem 634(1–3):83–88
Notario R (2014) A computational study of 2-selenobarbituric acid: conformational analysis, enthalpy of formation, acidity and basicity. Procedia Comput Sci 29:1356–1365
Becke AD (1997) Density-functional thermochemistry. V. Systematic optimization of exchange-correlation functionals. J Chem Phys 107(20):8554–8560
Marenich AV, Cramer CJ, Truhlar DG (2009) Universal solvation model based on solute electron density and on a continuum model of the solvent defined by the bulk dielectric constant and atomic surface tensions. J Phys Chem B 113(18):6378–6396
Fukui K (1981) The path of chemical reactions-the IRC approach. Acc Chem Res 14(12):363–368
Nemeryuk M, Arutyunyan T, Shatukhina E, Nerseyan N, Anisimova O, Soloveva N, Peresleni E, Sheinker YN, Sokol O, Kazakova V (1989) 6, 7, 8, 9-Tetrahydrodipyrimido [4, 5-b][4′, 5′-e][1, 4] thiazine derivatives. Synthesis and structure. Chem Heterocycl Compd 25(2):214–220
Sweidan K, Dayyih WA, AlDamen MA, Mallah E, Arafat T, El-Abadelah MM, Salih H, Voelter W (2017) Ring opening of cyclobutane in 1, 3-dimethyl-5-methylenebarbituric acid dimer by various nucleophiles. Z Naturforschung B 72(5):377–382
Sweidan K, AlDamen MA, Sinnokrotb MO, Al-Sheikhc A, Mubaraka MS (2017) Stabilization of meldrum's acid dimer and 1, 3-dimethylbarbituric acid trimer: a theoretical study. Jordan J Chem 12(1):1–10
AlDamen MA, Mubarak MS (2013) Theoretical and experimental study of lone pair interactions in THF/chloranilic acid system. Struct Chem 24(1):215–222
Manivel P, Prabakaran K, Krishnakumar V, Nawaz Khan F-R, Maiyalagan T (2014) Thiourea-mediated regioselective synthesis of symmetrical and unsymmetrical diversified thioethers. Ind Eng Chem Res 53(19):7866–7870
Mallah E, Al-Sheikh A, Sweidan K, Abu Dayyih W, Steimann M (2015) Crystal structure of 5-[bis (methylsulfonyl) methyl]-1, 3-dimethyl-5-(methylsulfonyl) pyrimidine-2, 4, 6 (1H, 3H, 5H)-trione. Acta Crystallogr E 71(1):o58–o59
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
10870_2020_846_MOESM1_ESM.docx
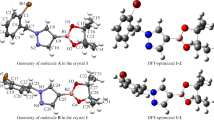
Additional Supporting Information may be found online in the supporting information tab for this article. X-ray crystallographic data for the structural analyses have been deposited with the Cambridge Crystallographic Data Centre (compound 8: CCDC No. 1981657). Potential energy diagram for steps A and B along with the Cartesians coordinates for all stationary points for proposed mechanisms and thermochemical data. CIF files containing complete information on the studied structures may be obtained free of charge from the Director, CCDC, 12 Union Road, Cambridge, CB2 1EZ, UK, Fax 0044-1223- 336033; E-mail:deposit@ccdc.cam.ac.uk or from the following web site: www.ccdc.cam.ac.uk/data_request/cif. Supplementary file1 (DOCX 1017 kb)
Rights and permissions
About this article
Cite this article
Sweidan, K., Almatarneh, M.H., AlDamen, M.A. et al. Understanding the Formation of 5-(Diethylammoniothio)-1,3-dimethylbarbituric Acid: Crystal Structure and DFT Studies. J Chem Crystallogr 51, 215–224 (2021). https://doi.org/10.1007/s10870-020-00846-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10870-020-00846-1