Abstract
Introduction
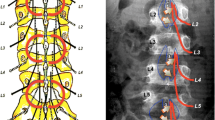
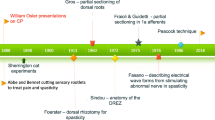
Spasticity is the result of an exaggeration of the monosynaptic muscle stretch reflex due to lesions affecting the central nervous system, in particular an upper motor neuron lesion. Selective dorsal rhizotomy (SDR) is a surgical technique developed to treat spastic diplegia, one of the common forms of cerebral palsy, resulting from the lack of supraspinal inhibitory controls. The aim of SDR is to identify and cut a critical amount of the sensory rootlets, in particular those contributing the most to spasticity, in order to relieve the patient from lower limb spasticity while preserving motor strength and sphincter control. Various surgical techniques to perform SDR have been proposed over time. Similarly, intraoperative neurophysiology (ION)—first introduced by Fasano and colleagues in 1976—is a safe and effective tool to guide the surgeon in the procedure of SDR, but different ION strategies are used by different authors, and the value of ION itself has been questioned.
Methods
The purpose of this paper is to review the anatomo-physiological background of SDR, the historical development of the surgical technique, and the essential principles of ION.
Results
While some surgeons privilege a single-level approach and others a multi-level approach, nowadays, there are still neither agreement nor guidelines on the percentage of roots to be cut. Rather, a tailored approach based on both the preoperative functional status as well as intraoperative ION findings seems reasonable. ION is considered not essential to decide the percentage of roots to cut, but it assists to distinguish between ventral and dorsal roots, and to preserve sphincterial function, whenever S2 rootlets are included in SDR.
Conclusions
To optimize the balance between reduction of spasticity and preservation of motor strength while minimizing the neurological damage remains the main goal of SDR.




Similar content being viewed by others
References
Abbott R (2007) Editorial on ‘The management of childhood hypertonia’. Childs Nerv Syst 23(9):937–941
Abbott R (1996) Sensory rhizotomy for the treatment of childhood spasticity. J Child Neurol 11(Suppl. 1):36–42
Aquilina K, Graham D, Wimalasundera N (2015) Selective dorsal rhizotomy: an old treatment re-emerging. Arch Dis Child 100(8):798–802
Bax M, Goldstein M, Rosenbaum P, Levinton A, Paneth N (2005) Proposed definition and classification of cerebral palsy. Dev Med Child Neurol 47(April):571–576
Cohen AR, Webster HC (1991) How selective is selective posterior rhizotomy? Surg Neurol 35(4):267–272
Enslin JMN, Langerak NG, Fieggen AG (2019) The evolution of selective dorsal rhizotomy for the management of spasticity. Neurotherapeutics 16(1):3–8
Fasano VA, Barolat-Romana G, Ivaldi A, Sguazzi A (1976) La radicotomie postérieure fonctionnelle dans le traitement de la spasticité cérébrale Premieres observations sur la stimulation électrique peropératoire des racines postérieures, et leur utilisation dans le choix des racines à sectionner. Neurochirurgie 22(1):23–24
Filloux FM (1996) Neuropathophysiology of movement disorders in cerebral palsy. J Child Neurol 11(Suppl. 1):S5–S12
Foerster O (1913) On the indication and results of the excision of posterior roots in men. Surg Gynecol Obstet 16:463–475
Freud S (1897) Die infantile Cerebrallähmung. In: Specielle Pathologie und Therapie Bd IX, Teil III, Holder ed (Wien), pp. 1–327
Fukuhara T, Nakatsu D, Namba Y, Yamadori I (2011) Histological evidence of intraoperative monitoring efficacy in selective dorsal rhizotomy. Childs Nerv Syst 27(9):1453–1458
Georgoulis G, Brînzeu A, Sindou M (2018) Dorsal rhizotomy for children with spastic diplegia of cerebral palsy origin: usefulness of intraoperative monitoring. J Neurosurg Pediatr 22(1):89–101
Graham D, Aquilina K, Cawker S, Paget S, Wimalasundera N (2016) Single-level selective dorsal rhizotomy for spastic cerebral palsy. J Spine Surg 2(3):195–201
Graham D, Aquilina K, Mankad K, Wimalasundera N (2018) Selective dorsal rhizotomy: current state of practice and the role of imaging. Quant Imaging Med Surg 8(2):209–218
Gros C, Ouaknine G, Vlahovitch B, Frerebeau P (1967) La radicotomie sélective postérieure dans le traitement neuro-chirurgical de l’hypertonie pyramidale. Neurochirurgie 13:505–518
Hultborn H (2006) Spinal reflexes, mechanisms and concepts : from Eccles to Lundberg and beyond. Prog Neurobiol 78:215–232
Kothbauer KF, Deletis V (2010) Intraoperative neurophysiology of the conus medullaris and cauda equina. Childs Nerv Syst 26(2):247–253
Lang FF, Deletis V, Cohen HW, Velasquez L, Abbott R (1994) Inclusion of the S2 dorsal rootlets in functional posterior rhizotomy for spasticity in children with cerebral palsy. Neurosurgery 34(5):847–853
Langerak NG, Lamberts RP, Fieggen AG, Peter JC, Peacock WJ, Vaughan CL (2007) Selective dorsal rhizotomy: long-term experience from Cape Town. Childs Nerv Syst 23(9):1003–1006
Lazareff JA, Garcia-Mendez MA, De Rosa R, Olmstead C (1999) Limited (L4-S1, L5-S1) selective dorsal rhizotomy for reducing spasticity in cerebral palsy. Acta Neurochir 141(7):743–752
Little WJ (1966) On the influence of abnormal parturition, difficult labours, premature birth, and asphyxia neonatorum, on the mental and physical condition of the child, especially in relation to deformities. Clin Orthop Relat Res 46:7–22
Logigian EL (1994) H reflex studies in cerebral palsy patient undergoing partial dorsal rhizotomy. Muscle Nerve 17(5):539–549
MacKinnon CD (2018) Sensorimotor anatomy of gait, balance, and falls. Handb Clin Neurol 159:3–26
MacLennan AH, Thompson SC, Gecz J (2015) Cerebral palsy: causes, pathways, and the role of genetic variants. Am J Obstet Gynecol 213(6):779–788
McLaughlin J, Bjornson K, Temkin N, Steinbok P, Wright V, Reiner A, Roberts T, Drake J, O'Donnell M, Rosenbaum P, Barber J, Ferrel A (2002) Selective dorsal rhizotomy: meta-analysis of three randomized controlled trials. Dev Med Child Neurol 44(3):17–25
Mittal S, Farmer JP, Poulin C, Silver K (2001) Reliability of intraoperative electrophysiological monitoring in selective posterior rhizotomy. J Neurosurg 95(1):67–75
Moreno De Luca A, Ledbetter DH, Martin CL (2012) Genetic insights into the causes and classification of the cerebral palsies. Lancet Neurol 11:283–292
Morota N (2019) Clinically practical formula for preoperatively estimating the cutting rate of the spinal nerve root in a functional posterior rhizotomy. Childs Nerv Syst 35(4):665–672
Morota N (2007) Functional posterior rhizotomy: the Tokyo experience. Childs Nerv Syst 23(9):1007–1014
Netter F (2014) Atlas of human anatomy. 6th ed, Edra
Osler SW (1889) The cerebral palsies of children: a clinical study for the infirmary for nervous diseases, classics of neurology and neurosurgery Library, Philadelphia
Panteliadis CP (2018) Cerebral palsy - a multidisciplinary approach, 3rd edn. Springer International Publishing, pp 35–47
Papadelis C, Kaye H, Shore B, Snyder B, Grant PE, Rotenberg A (2019) Maturation of corticospinal tracts in children with hemiplegic cerebral palsy assessed by diffusion tensor imaging and transcranial magnetic stimulation. Front Hum Neurosci 13(July):1–9
Park TS, Dobbs MB, Cho J (2018) Evidence supporting selective dorsal rhizotomy for treatment of spastic cerebral palsy. Cureus 10(10):e3466
Park TS, Johnston JM (2006) Surgical techniques of selective dorsal rhizotomy for spastic cerebral palsy.Technical note. Neurosurg Focus 21(2):1–6
Peacock WJ, Aren LJ, Berman B (1987) Cerebral palsy spasticity. Selective posterior rhizotomy. Pediatr Neurosci 13:61–66
Sherrington CS (1898) Decerebrate rigidity and reflex coordination of movements. J Physiol 22:319–327
Sindou M, Georgoulis G, Mertens P (2014) Neurosurgery for spasticity- a practical guide for treating children and adults, 1st edn. Springer-Verlag, Wien
Sindou M (1995) Microsurgical DREZotomy (MDT) for pain, spasticity, and hyperactive bladder: a 20-year experience. Acta Neurochir 137:1–5
Sindou M (2019) Surgery in the dorsal root entry zone for spasticity. In: Lozano AM, Gildenberg PL, Tasker RR (eds) Textbook of stereotactic and functional neurosurgery. Springer, Berlin, pp 1–26
Smyth MD, Peacock WJ (2000) The surgical treatment of spasticity. Muscle Nerve 23:153–163
Steinbok P, Gustavsson B, Kestle JRW, Reiner A, Cochrane DD (1995) Relationship of intraoperative electrophysiological criteria to outcome after selective functional posterior rhizotomy. J Neurosurg 83(1):18–26
Steinbok P, Keyes R, Langill L, Cochrane DD (1994) The validity of electrophysiological criteria used in selective functional posterior rhizotomy for treatment of spastic cerebral palsy. J Neurosurg 81(3):354–361
Steinbok P, Langill L, Cochrane DD, Keyes R (1992) Observations on electrical stimulation of lumbosacral nerve roots in children with and without lower limb spasticity. Childs Nerv Syst 8:376–382
Steinbok P, Reiner A, Beauchamp RD, Cochrane DD, Keyes R (1992) Selective functional posterior rhizotomy for treatment of spastic cerebral palsy in children. Pediatr Neurosurg 18(1):34–42
Steinbok P, Tidemann AJ, Miller S, Mortenson P, Bowen-Roberts T (2009) Electrophysiologically guided versus non-electrophysiologically guided selective dorsal rhizotomy for spastic cerebral palsy: a comparison of outcomes. Childs Nerv Syst 25(9):1091–1096
Steinbok P (2007) Selective dorsal rhizotomy for spastic cerebral palsy: a review. Childs Nerv Syst 23(9):981–990
Storrs BB, Nishida T (1988) Use of ‘H’ reflex recovery curve in selective posterior rhizothomy. Pediatr Neurosci 14:120–123
Trompetto C, Marinelli L, Mori L, Pelosin E, Currà A, Molfetta L, Abbruzzese G (2014) Pathophysiology of spasticity: implications for neurorehabilitation. Biomed Res Int 2014:1–8. https://doi.org/10.1155/2014/354906
Turner RP (2009) Neurophysiologic intraoperative monitoring during selective dorsal rhizotomy. J Clin Neurophysiol 26(2):82–84
Wang KK, Munger ME, Chen BP, Novacheck TF (2018) Selective dorsal rhizotomy in ambulant children with cerebral palsy. J Child Orthop 12(5):413–427
Xiao B, Constatntini S, Browd SR, Zhan Q, Jiang W, Mei R (2019) The role of intra-operative neuroelectrophysiological monitoring in single-level approach selective dorsal rhizotomy. Childs Nerv Syst:1–9. https://doi.org/10.1007/s00381-019-04408-5
Zhou M, Wang W, Huang H, Zhu G, Chen Y, Zhou C (2010) Microsurgical anatomy of lumbosacral nerve rootlets for highly selective rhizotomy in chronic spinal cord injury. Anat Rec 293:2123–2128
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Pasquali, C., Deletis, V. & Sala, F. Selective dorsal rhizotomy: functional anatomy of the conus-cauda and essentials of intraoperative neurophysiology. Childs Nerv Syst 36, 1907–1918 (2020). https://doi.org/10.1007/s00381-020-04746-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00381-020-04746-9