Article contents
Mixed phase ytterbium silicate environmental-barrier coating materials for improved calcium–magnesium–alumino-silicate resistance
Published online by Cambridge University Press: 06 July 2020
Abstract
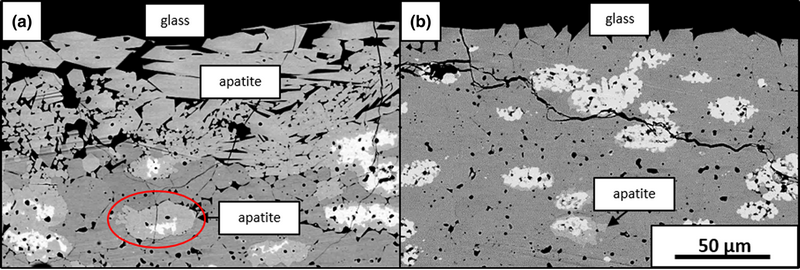
Calcium–magnesium–alumino-silicate (CMAS) reaction and infiltration behavior were studied in phase pure and mixed phase ytterbium silicate environmental-barrier coating (EBC) materials at 1300 °C. Phase pure Yb2Si2O7 (YbDS) was infiltrated by CMAS via grain boundaries/pores, resulting in loss of its structural integrity. Phase pure Yb2SiO5 (YbMS) reacted with CMAS to form either apatite (Ca2Yb8(SiO4)6O2) or YbDS, depending on the initial glass composition. Both reactions in YbMS slowed infiltration kinetics considerably compared to YbDS. Samples having a YbDS matrix with controlled amounts and dispersions of YbMS were also investigated as a model for air plasma spray coatings. Samples containing ≥20 vol% coarse YbMS showed dramatically improved infiltration behavior compared to phase pure YbDS. YbDS samples containing a fine dispersion of YbMS displayed a new mode of CMAS attack in which glass spread on the sample surfaces. The results of this study suggest that EBC phase compositions and microstructures may be tailored for optimized CMAS resistance.
- Type
- Article
- Information
- Journal of Materials Research , Volume 35 , Issue 17: Focus Issue: Sand-phobic Thermal/Environmental Barrier Coatings for Gas Turbine Engines , 14 September 2020 , pp. 2358 - 2372
- Copyright
- Copyright © The Author(s), 2020, published on behalf of Materials Research Society by Cambridge University Press
References
- 12
- Cited by




