Abstract
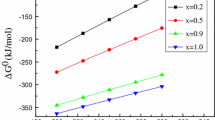
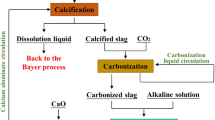
The rapid development of the alumina industry in China has led to the depletion of high-quality bauxite resources. The proposed calcification-carbonation method (CCM) can convert the middle silicon of bauxite into CaO · SiO2 and CaCO3, thus enabling clean and efficient utilization of low-grade resources. Since kaolinite is the main silicon-bearing mineral in low-grade bauxite, the transformation characteristics of kaolinite in the CCM process are investigated in this research. The experimental results indicate that temperature has a significant effect on both the calcification process and the carbonation process. The theoretical alumina-to-silica ratio (A/S) in the reacted residue can be lowered to 0.55 using the CCM process used to treat gibbsite, resulting in a recovery ratio of alumina approximately 15% greater than that of the Bayer process. More than 65% of the alumina can be recovered through the CCM. The Na2O content in the slag from the CCM meets the requirements of the cement industry, and cleaner production of alumina can be enabled by the CCM.







Similar content being viewed by others
REFERENCES
Liu, Z., Li, D., Ma, W., et al., Sulfur removal by adding aluminum in the Bayer process of high-sulfur bauxite, Miner. Eng., 2018, no. 119, pp. 76–81.
Wang, Y., et al., Application of tricalcium aluminate instead of lime for the recovery of aluminum in middle-low grade bauxite in calcification-carbonization process, in Light Metals, The Minerals, Metals & Materials Series, Ratvik, A., Ed., Cham: Springer, 2017, pp. 61–66.
Zhang, Y.-Y., Wei, L., Qi, Y.-h., et al., Recovery of iron and calcium aluminate slag from high-ferrous bauxite by high-temperature reduction and smelting process, Int. J. Miner., Metall. Mater., 2016, no. 8. pp. 881–890.
Lu, G., Zhang, T., Zhu, X., et al., Calcification-carbonation method for cleaner alumina production and CO2 utilization, JOM, 2014, no. 9, pp. 1616–1621.
You, S., Zhang, Y., Cao, S., et al., Crystallization of monosodium aluminate hydrate from a concentrated aluminate solution containing silica, Hydrometallurgy, 2012, no. 4, pp. 104–107.
Li, X., Huang, X., Qi, T., et al., Preliminary results on selective surface magnetization and separation of alumina-/silica-bearing minerals, Miner. Eng., 2015, vol. 81, pp. 135–141.
Ruan, S., Shi, L., Li, J., et al., Desilication of hematite, goethite and iron powder seeded low alumina to caustic liquors, Hydrometallurgy, 2017, vol. 169, pp. 297–305.
Webster, N.A.S., Loan, M.J., Madsen, I.C., et al., An investigation of the mechanisms of goethite, hematite and magnetite-seeded Al(OH)3, precipitation from synthetic Bayer liquor, Hydrometallurgy, 2011, no. 1, pp. 72–79.
Power, G., Gräfe, M., and Klauber, C., Bauxite residue issues: I. Current management, disposal and storage practices, Hydrometallurgy, 2011, no. 1, pp. 33–45.
Radomirovic, T., Smith, P., Southam, D., et al., Crystallization of sodalite particles under Bayer-type conditions, Hydrometallurgy, 2013, vol. 137, pp. 84–91.
Zhang, N., Liu, X., Sun, H., et al., Pozzolanic behavior of compound-activated red mud-coal gangue mixture, Cem. Concr. Res., 2011, no. 3, pp. 270–278.
Borges, A.J.P., Hauser-Davis, R.A., et al., Cleaner red mud residue production at an alumina plant by applying experimental design techniques in the filtration stage, J. Cleaner Prod., 2011, no. 15, pp. 1763–1769.
Brunori, C., Cremisini, C., Massanisso, P., et al., Reuse of a treated red mud bauxite waste: studies on environmental compatibility, J. Hazard. Mater., 2005, no. 1, pp. 55–63.
Liu, W., Yang, J., and Xiao, B., Application of Bayer red mud for iron recovery and building material production from alumosilicate residues, J. Hazard. Mater., 2009, no. 1, pp. 474–478.
Kumar, A. and Kumar, S., Development of paving blocks from synergistic use of red mud and fly ash using geopolymerization, Constr. Build. Mater., 2013, no. 32, pp. 865–871.
Xuan, D., Zhan, B., Poon, C.S., et al., Innovative reuse of concrete slurry waste from ready-mixed concrete plants in construction products, J. Hazard. Mater., 2016, no. 312, pp. 65–72.
Hajjaji, W., et al., Composition and technological properties of geopolymers based on metakaolin and red mud, Mater. Des., 2013, no. 24, pp. 648–654.
Gelencsér, A., et al., The red mud accident in Ajka (Hungary): characterization and potential health effects of fugitive dust, Environ. Sci. Technol., 2011, no. 4, pp. 1608–1615.
Yang, C., Gui, W., Kong, L., et al., A two-stage intelligent optimization system for the raw slurry preparing process of alumina sintering production, Eng. Appl. Artif. Intell., 2009, no. 4, pp. 786–795.
Yang, J. and Bo, X., Development of unsintered construction materials from red mud wastes produced in the sintering alumina process, Constr. Build. Mater., 2008, no. 12, pp. 2299–2307.
Lu, G., Zhang, T., Ma, L., et al., Utilization of Bayer red mud by a calcification-carbonation method using calcium aluminate hydrate as a calcium source, Hydrometallurgy, 2019, no. 188, pp. 248–255.
Li, R., Zhang, T., Liu, Y., et al., Calcification-carbonation method for red mud processing, J. Hazard. Mater., 2016, no. 316, pp. 94–101.
Xie, L., Zhang, T., Lv, G., et al., Direct calcification-carbonation method for processing of Bayer process red mud, Russ. J. Non-Ferrous Met., 2018, no. 59, pp. 142–147.
Lu, G., Zhang, T., Guo, F., et al., Clean and efficient utilization of low-grade high-iron sedimentary bauxite via calcification-carbonation method, Hydrometallurgy, 2019, no. 187, pp. 195–202.
Lu, G., Zhang, T., Zheng, C., et al., The influence of the silicon saturation coefficient on a calcification-carbonation method for clean and efficient use of bauxite, Hydrometallurgy, 2017, no. 174, pp. 97–104.
Lager, G.A., Nipko, J.C., and Loong, C.K., Inelastic neutron scattering study of the (O4H4) substitution in garnet, Phys. B (Amsterdam, Neth.), 1997, nos. 241–243, pp. 406–408.
Mercury, J.M.R., et al., Solid-state Al and Si NMR investigations on Si-substituted hydrogarnets, Acta Mater., 2007, no. 4, pp. 1183–1191.
Zhang, R., Zheng, S., Ma, S., et al., Recovery of alumina and alkali in Bayer red mud by the formation of andradite-grossular hydrogarnet in hydrothermal process, J. Hazard. Mater., 2011, no. 3, pp. 827–835.
Nikolaev, I.V., Kirov, S.S., Vorob’ev, I.B., et al., Applicability of hydrogarnet technology for complex processing of Indian condalites, Russ. J. Non-Ferrous Met., 2011, vol. 52, pp. 150–156.
Locock, A.J., An Excel spreadsheet to recast analyses of garnet into end-member components, and a synopsis of the crystal chemistry of natural silicate garnets, Comput. Geosci., 2008, no. 12, pp. 1769–1780.
FUNDING
We acknowledge the National Natural Science Foundation of China (nos. 51874078, U1710257, U1202274), State Key Laboratory of Pressure Hydrometallurgical Technology of Associated Nonferrous Metal Resources (YY2016006), the Fundamental Research Funds for the Central Universities (N182505038), and Shenyang Science and Technology Project (17-500-8-01, Z18-5-022).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare to have no conflict of interest.
About this article
Cite this article
Zimu Zhang, Lu, G., Chen, Y. et al. Alumina Extraction from Kaolinite via Calcification-Carbonation Process. Russ. J. Non-ferrous Metals 61, 248–256 (2020). https://doi.org/10.3103/S1067821220030207
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.3103/S1067821220030207