Abstract
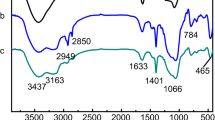
In this study, the preparation of molecularly imprinted polymers for bilobalide (BBMIPs) was successfully achieved by bulk polymerization with methacrylamide (MAM), trimethylolpropane triacrylate (TMPTA), and acetonitrile (ACN) as functional monomer, cross-linker, and solvent, respectively. After Gaussian software simulation and single factor experiments, the prepared MIPs with a molar ratio of 1:4:15 for BB-MAM-TMPTA were systematically characterized. The hydrogen bonding interaction between BB and MAM was confirmed by a combination of FTIR and NMR analysis. Thermal gravimetric analysis results displayed that MIPs have excellent thermal stability under high temperature. Additionally, the average pore size and surface area of MIPs were found to be higher than those of NIPs through nitrogen adsorption results. The results of static adsorption and kinetic adsorption suggested that the adsorption equilibrium concentration was 0.6 mg/mL and the equilibrium time was 5 h, and the Langmuir and pseudo-second-order kinetic models were proven to fit with static and kinetic adsorption behaviors, respectively. Meanwhile, the selective adsorption study revealed that MIPs show high adsorption and great selectivity towards BB in comparison with other substances having similarly structure. MIPs also possessed a good performance on reusability, maintaining a high recovery rate after being reused 5 times. The application experiment further indicated that MIPs can effectively separate BB from low purity samples. Therefore, the prepared MIPs had a great potential for BB separation.









Similar content being viewed by others
References
Atzori C, Bruno A, Chichino G, Bombardelli E, Scaglia M, Ghione M (1993) Activity of bilobalide, a sesquiterpene from Ginkgo biloba, on Pneumocystis carinii. Antimicrob Agents Chemother 37(7):1492–1496. https://doi.org/10.1128/aac.37.7.1492
Huang SH, Duke RK, Chebib M, Sasaki K, Wada K, Johnston GAR (2003) Bilobalide, a sesquiterpene trilactone from Ginkgo biloba, is an antagonist at recombinant alpha(1) beta(2)gamma(2L) GABA(A) receptors. Eur J Pharmacol 464(1):1–8. https://doi.org/10.1016/s0014-2999(03)01344-x
Zhou W, Chai H, Lin PH, Lumsden AB, Yao QZ, Chen CY (2004) Clinical use and molecular mechanisms of action of extract of Ginkgo biloba leaves in cardiovascular diseases. Cardiovasc Drug Rev 22(4):309–319
Ahlemeyer B, Krieglstein J (2003) Pharmacological studies supporting the therapeutic use of Ginkgo biloba extract for Alzheimer’s disease. Pharmacopsychiatry 36:S8–S14
Loew D (2002) Value of Ginkgo biloba in treatment of Alzheimer dementia (Stellenwert von Ginkgo biloba in der Behandlung der Alzheimer-Demenz.). Wiener medizinische Wochenschrift (1946) 152 (15–16):418–422. doi:https://doi.org/10.1046/j.1563-258X.2002.02065.x
Qiu F, Friesen JB, McAlpine JB, Pauli GF (2012) Design of countercurrent separation of Ginkgo biloba terpene lactones by nuclear magnetic resonance. J Chromatogr A 1242:26–34. https://doi.org/10.1016/j.chroma.2012.03.081
Gao D, Fu QF, Wang LJ, Wang DD, Zhang KL, Yang FQ, Xia ZN (2017) Molecularly imprinted polymers for the selective extraction of tiliroside from the flowers of Edgeworthia gardneri (wall.) Meisn. J Sep Sci 40(12):2629–2637. https://doi.org/10.1002/jssc.201700240
Chen LX, Xu SF, Li JH (2011) Recent advances in molecular imprinting technology: current status, challenges and highlighted applications. Chem Soc Rev 40(5):2922–2942. https://doi.org/10.1039/c0cs00084a
Meng MJ, He MQ (2016) Highly effective surface molecularly imprinted polymer for the solid-phase extraction of dihydroquercetin from Prince’s-feather Fruit sample. Sep Sci Technol 51(6):917–928. https://doi.org/10.1080/01496395.2016.1142561
Shao HK, Zhao LG, Chen J, Zhou HT, Huang ST, Li K (2015) Preparation, characterization and application of molecularly imprinted monolithic column for hesperetin. J Pharm Biomed Anal 111:241–247. https://doi.org/10.1016/j.jpba.2015.04.006
Yusof NA, Ab Rahman SK, Hussein MZ, Ibrahim NA (2013) Preparation and characterization of molecularly imprinted polymer as SPE sorbent for melamine isolation. Polymers 5(4):1215–1228. https://doi.org/10.3390/polym5041215
Jinn WS, Shin MK, Kang B, Oh S, Moon CE, Mun B, Ji YW, Lee HK, Haam S (2019) A visually distinguishable light interfering bioresponsive silica nanoparticle hydrogel sensor fabricated through the molecular imprinting technique. J Mater Chem B 7(45):7120–7128. https://doi.org/10.1039/c9tb01579e
Asghar N, Mustafa G, Yasinzai M, Al-Soud YA, Lieberzeit PA, Latif U (2019) Real-time and online monitoring of glucose contents by using molecular imprinted polymer-based IDEs sensor. Appl Biochem Biotechnol 189(4):1156–1166. https://doi.org/10.1007/s12010-019-03049-3
Li SJ, Ge Y, Tiwari A, Wang SQ, Turner APF, Piletsky SA (2011) ‘On/off’-switchable catalysis by a smart enzyme-like imprinted polymer. J Catal 278(2):173–180. https://doi.org/10.1016/j.jcat.2010.11.011
Wulff G (2002) Enzyme-like catalysis by molecularly imprinted polymers. Chem Rev 102(1):1–27. https://doi.org/10.1021/cr980039a
Chen FF, Wang R, Shi YP (2012) Molecularly imprinted polymer for the specific solid-phase extraction of kirenol from Siegesbeckia pubescens herbal extract. Talanta 89:505–512. https://doi.org/10.1016/j.talanta.2011.12.080
Wu R, Shui L, Wang S, Song Z, Tai F (2016) Bilobalide alleviates depression-like behavior and cognitive deficit induced by chronic unpredictable mild stress in mice. Behav Pharmacol 27(7):596–605. https://doi.org/10.1097/fbp.0000000000000252
Chen LZ, Wang RY, Cui L, Wang X, Wang LL, Song FY, Ji WH (2018) Preparation of five high-purity iridoid glycosides from Gardenia jasminoides Eills by molecularly imprinted solid-phase extraction integrated with preparative liquid chromatography. J Sep Sci 41(13):2759–2766. https://doi.org/10.1002/jssc.201800086
Feng YG, Liu Q, Ye LF, Wu QZ, He JF (2017) Ordered macroporous quercetin molecularly imprinted polymers: preparation, characterization, and separation performance. J Sep Sci 40(4):971–978. https://doi.org/10.1002/jssc.201601011
Zhu HB, Wang YZ, Yuan Y, Zeng HA (2011) Development and characterization of molecularly imprinted polymer microspheres for the selective detection of kaempferol in traditional Chinese medicines. Anal Methods 3(2):348–355. https://doi.org/10.1039/c0ay00578a
Khan S, Bhatia T, Trivedi P, Satyanarayana GNV, Mandrah K, Saxena PN, Mudiam MKR, Roy SK (2016) Selective solid-phase extraction using molecularly imprinted polymer as a sorbent for the analysis of fenarimol in food samples. Food Chem 199:870–875. https://doi.org/10.1016/j.foodchem.2015.12.091
Mohajeri SA, Karimi G, Aghamohammadian J, Khansari MR (2011) Clozapine recognition via molecularly imprinted polymers; bulk polymerization versus precipitation method. J Appl Polym Sci 121(6):3590–3595. https://doi.org/10.1002/app.34147
Razwan SM, Jin Y, Kong GH, Li HF, Lin JM (2014) Molecularly imprinted polymer for pre-concentration of esculetin from tobacco followed by the UPLC analysis. Sci China-Chem 57(12):1751–1759. https://doi.org/10.1007/s11426-014-5180-1
Fuchs Y, Soppera O, Haupt K (2012) Photopolymerization and photostructuring of molecularly imprinted polymers for sensor applications-a review. Anal Chim Acta 717:7–20. https://doi.org/10.1016/j.aca.2011.12.026
Golsefidi MA, Es’haghi Z, Sarafraz-Yazdi A (2012) Design, synthesis and evaluation of a molecularly imprinted polymer for hollow fiber-solid phase microextraction of chlorogenic acid in medicinal plants. J Chromatogr A 1229:24–29. https://doi.org/10.1016/j.chroma.2012.01.019
Karim K, Breton F, Rouillon R, Piletska EV, Guerreiro A, Chianella I, Piletsky SA (2005) How to find effective functional monomers for effective molecularly imprinted polymers? Adv Drug Deliv Rev 57(12):1795–1808. https://doi.org/10.1016/j.addr.2005.07.013
de Barros LA, Pereira LA, Custodio R, Rath S (2014) A novel computational approach for development of highly selective fenitrothion imprinted polymer: theoretical predictions and experimental validations. J Braz Chem Soc 25(4):619–628. https://doi.org/10.5935/0103-5053.20140009
Fonseca MC, Nascimento Jr CS, Borges KB (2016) Theoretical investigation on functional monomer and solvent selection for molecular imprinting of tramadol. Chem Phys Lett 645:174–179. https://doi.org/10.1016/j.cplett.2015.12.061
Tadi KK, Motghare RV, Ganesh V (2015) Electrochemical detection of epinephrine using a biomimic made up of hemin modified molecularly imprinted microspheres. RSC Adv 5(120):99115–99124. https://doi.org/10.1039/c5ra16636e
M.J. Frisch, G.W. Trucks, H.B. Schlegel, G.E. Scuseria, M.A. Robb(2016) Gaussian 16, Revision A.03, Gaussian, Inc.,Wallingford CT
Li HH, Zhang WC, Wu ZY, Huang XS, Hui AL, He YW, Wang HY (2020) Theoretical design, preparation, and evaluation of Ginkgolide B molecularly imprinted polymers. J Sep Sci 43(2):514–523. https://doi.org/10.1002/jssc.201900675
Funding
The Gaussian 2016 software package was provided by Professor Xiaorong Wang, School of Petrochemical Engineering, Liaoning Shihua University. This study was financially supported by Anhui Provincial Natural Science Foundation (no. 1808085MC77), Key Project of Science and Technology Research and Development of Anhui Province (no. 1704a07020094), Fundamental Research Funds for the Central Universities of China (nos. JZ2019YYPY0028, PA2019GDPK0084, and PA2019GDZC0099), and Enterprise Cooperation Project (no. W2017JSKF0682).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 1290 kb)
Rights and permissions
About this article
Cite this article
Huang, X., Zhang, W., Wu, Z. et al. Computer simulation aided preparation of molecularly imprinted polymers for separation of bilobalide. J Mol Model 26, 198 (2020). https://doi.org/10.1007/s00894-020-04460-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-020-04460-y