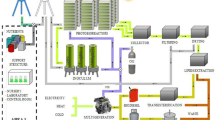
Abstract
The purpose of this study was to determine the biogas potential of biomass produced by microbiotic communities developed under natural conditions in freshwater systems such as ponds incorporated into agricultural landscapes. Natural communities of microalgae were collected from a small eutrophicated pond where dominant species were euglenoids (Lepocinclis species). Cyanobacterial communities dominated by Lyngbya species were taken from a domestic aquarium and cultivated under makeshift conditions. Experiments were done using dairy cow manure (DCM) for codigestion with natural communities of microalgae (MDM) and cyanobacteria (CDM) and conducted during 42 days in thermophilic regime. The total biogas yields were 421.40 and 383.34 mL/g volatile solids (VS), while the average methane contents were 63.97 and 64.06% for MDM and CDM, respectively. Our results indicate that the natural communities of microalgae and cyanobacteria used in this study possess the potential for biogas production, which is, in comparison with particular algal and cyanobacterial strains cultivated under strictly controlled cultivation conditions, more promising. Therefore, this study aims to motivate further investigations into the diverse natural communities of microalgae and cyanobacteria and pretreatments that are environmentally friendly and cost-effective and will eventually enhance small-scale biogas production on agricultural farms.



Similar content being viewed by others
Abbreviations
- DCM:
-
Dairy cow manure
- MDM:
-
Microalgae and DCM mixture
- CDM:
-
Cyanobacteria and DCM mixture
- TS:
-
Total solids
- VS:
-
Volatile solids
- TOC:
-
Total organic carbon
- TN:
-
Total extractable nitrogen
- GC:
-
Gas chromatograph
- SD:
-
Standard deviation
References
Milledge, J. J., Nielsen, B. V., Maneein, S., & Harvey, P. J. (2019). A brief review of anaerobic digestion of algae for bioenergy. Energies, 12(6), 1166. https://doi.org/10.3390/en12061166.
Milledge, J. J., & Harvey, P. J. (2016). Potential process ‘hurdles’ in the use of macroalgae as feedstock for biofuel production in the British Isles. Journal of Chemical Technology and Biotechnology, 91(8), 2221–2234. https://doi.org/10.1002/jctb.5003.
Córdova, O., Passos, F., & Chamy, R. (2019). Enzymatic pretreatment of microalgae: cell wall disruption, biomass solubilisation and methane yield increase. Applied Biochemistry and Biotechnology, 189(3), 787–797. https://doi.org/10.1007/s12010-019-03044-8.
Luo, T., Zhu, N., Shen, F., Long, E., Long, Y., Chen, X., & Mei, Z. (2016). A case study assessment of the suitability of small-scale biogas plants to the dispersed agricultural structure of China. Waste and Biomass Valorization, 7(5), 1131–1139. https://doi.org/10.1007/s12649-016-9487-3.
Hjort-Gregersen, K. (2015). Market overview micro scale digesters, BioEnergy Farm II publication. Denmark: AgroTech A/S.
Mudimu, O., Rybalka, N., Bauersachs, T., Born, J., Friedl, T., & Schulz, R. (2014). Biotechnological screening of microalgal and cyanobacterial strains for biogas production and antibacterial and antifungal effects. Metabolites, 4(2), 373–393. https://doi.org/10.3390/metabo4020373.
Bohutskyi, P., & Bouwer, E. (2013). Biogas production from algae and cyanobacteria through anaerobic digestion: a review, analysis, and research needs. In J. W. Lee (Ed.), Advanced biofuels and bioproducts. New York: Springer Science Business Media.
Cavinato, C., Ugurlu, A., de Godos, I., Kendir, E., & Gonzalez-Fernandez, C. (2017). Biogas production from microalgae, in Microalgae-based biofuels and bioproducts: from feedstock cultivation to end-products (Gonzalez-Fernandez, C., Muñoz, R., eds.), Elsevier, pp. 155–182. https://doi.org/10.1016/B978-0-08-101023-5.00007-8
Wu, N., Moreira, C.M., Zhang, Y., Doan, N., Yang, S., Phlips, E.J., Svoronos, S.A., & Pullammanappallil, P.C. (2019). Techno-economic analysis of biogas production from microalgae through anaerobic digestion, in Anaerobic digestion (Banu, R. and Kannah, Y.R., eds.), IntechOpen.
Tiwari, A. & Marella, T.K. (2020). Algal biomass: potential renewable feedstock for biofuel production, in substrate analysis for effective biofuels production (Srivastava, N., Srivastava, M., Mishra. P.K., Gupta, V.K., eds.), Springer.
Ehimen, E. A., Sun, Z. F., Carrington, C. G., Birch, E. J., & Eaton-Rye, J. J. (2011). Anaerobic digestion of microalgae residues resulting from the biodiesel production process. Applied Energy, 88(10), 3454–3463. https://doi.org/10.1016/j.apenergy.2010.10.020.
Dębowski, M., Szwaja, S., Zieliński, M., Kisielewska, M., & Stańczyk-Mazanek, E. (2017). The influence of anaerobic digestion effluents (ADEs) used as the nutrient sources for Chlorella sp. cultivation on fermentative biogas production. Waste and Biomass Valorization, 8(4), 1153–1161. https://doi.org/10.1007/s12649-016-9667-1.
Duan, N., Ran, X., Li, R., Kougias, P. G., Zhang, Y., Lin, C., & Liu, H. (2018). Performance evaluation of mesophilic anaerobic digestion of chicken manure with algal digestate. Energies, 11(7), 1829. https://doi.org/10.3390/en11071829.
Ramos-Suárez, J. L., Martínez, A., & Carreras, N. (2014). Optimization of the digestion process of Scenedesmus sp. and Opuntia maxima for biogas production, Energy Convers. Manag., 88, 1263–1270. https://doi.org/10.1016/j.enconman.2014.02.064.
Marques, A. L., Pinto, F. P., Araújo, O. Q. F., & Cammarota, M. C. (2018). Assessment of methods to pretreat microalgal biomass for enhanced biogas production. Journal of Sustainable Development of Energy, Water environ. syst. 6, 394-404. https://doi.org/10.13044/j.sdewes.d5.0193.
Cirne, D. G., Paloumet, X., Bjornsson, L., Alves, M. M., & Mattiasson, B. (2007). Anaerobic digestion of lipid-rich waste - effects of lipid concentration, Renew. Energy, 32(6), 965–975. https://doi.org/10.1016/j.renene.2006.04.003.
Mussgnug, J. H., Klassen, V., Schlȕter, A., & Kruse, O. (2010). Microalgae as substrates for fermentative biogas production in a combined biorefinery concept. Journal of Biotechnology, 150(1), 51–56. https://doi.org/10.1016/j.jbiotec.2010.07.030.
Mendoza, Á., Morales, V., Sánchez-Bayo, A., Rodríguez-Escudero, R., González-Fernández, C., Bautista, L. F., & Vicente, G. (2020). The effect of the lipid extraction method used in biodiesel production on the integrated recovery of biodiesel and biogas from Nannochloropsis gaditana, Isochrysis galbana and Arthrospira platensis. Biochemical Engineering Journal, 154, 107428. https://doi.org/10.1016/j.bej.2019.107428.
Wilkie, A. C. (2005). Anaerobic digestion: biology and benefits, in Dairy manure management: treatment, handling, and community relations (pp. 63–72). Ithaca: Cornell University.
Hindak, F., Cyrus, Z., Marvan, P., Javornicky, P., Komarek, J., Ettl, H., Rosa, K., Sladečkova, A., Popovsky, J., Punčocharova, M., & Lhotsky, O. (1978). Slatkovodne Riasy. Bratislava: Slovenske Pedagogicke Nakladelstvo.
Anagostidis, K., & Komárek, J. (1988). Modern approach to the classification system of cyanophytes, 3: Oscillatories. Algological Studies/Archiv für Hydrobiologie, Suppl, 80, 327–472.
Utermöhl, H. (1958) Zur Vervollkommnung der Quantitativen Phytoplankton - Methodik Mitt IntVerein Theor Angew Limnol 9, 1-38.
APHA. (2005). Standard methods for the examination of water and wastewater. Washington: APHA.
ISO 14235 (1998) Soil quality – determination of organic carbon by sulfochromic oxidation
ISO 6974-4:2001 Natural gas - determination of composition with defined uncertainty by gas chromatography – part 4: determination of nitrogen, carbon dioxide and hydrocarbons C1 up to C5 and C6+ for a laboratory and on-line process application using two columns (ISO 6974-4:2000). https://www.iso.org/obp/ui/#iso:std:iso:6974:-4:ed-1:v1:en
Reynolds, C. S. (2006). The ecology of phytoplankton. Cambridge: Cambridge University Press.
Borics, G., Tóthmérész, B., Lukács, B. A., & Várbíró, G. (2012). Functional groups of phytoplankton shaping diversity of shallow lake ecosystems. Hydrobiologia, 698(1), 251–262. https://doi.org/10.1007/s10750-012-1129-6.
Stević, F., Mihaljević, M., & Špoljarić, D. (2013). Changes of phytoplankton functional groups in a floodplain lake associated with hydrological perturbations. Hydrobiologia, 709(1), 143–158. https://doi.org/10.1007/s10750-013-1444-6.
Poniewozik, M., & Juráň, J. (2018). Extremely high diversity of Euglenophytes in a small pond in eastern Poland, Plant Ecol. Evol., 151(1), 18–34. https://doi.org/10.5091/plecevo.2018.1308.
Chisti, Y. (2007). Biodiesel from microalgae. Biotechnology Advances, 25(3), 294–306. https://doi.org/10.1016/j.biotechadv.2007.02.001.
Wilkie, A. C., Edmundson, S. J., & Duncan, J. G. (2011). Indigenous algae for local bioresource production: phycoprospecting, Energy Sustain. Dev., 15(4), 365–371. https://doi.org/10.1016/j.esd.2011.07.010.
Nekano, Y., Urade, R., & Kitaoka, S. (1987). Isolation, purification and characterization of the pellicle of Euglena gracilisz. Journal of Biochemistry, 102(5), 1053–1063. https://doi.org/10.1093/oxfordjournals.jbchem.a122143.
Hudon, C., De Sève, M., & Cattaneo, A. (2014). Increasing occurrence of the benthic filamentous cyanobacterium Lyngbya wollei: a symptom of freshwater ecosystem degradation. Freshwater Science, 33(2), 606–618. https://doi.org/10.1086/675932.
Min, M., Hu, B., Mohr, M. J., Shi, A., Ding, J., Sun, Y., Jiang, Y., Fu, Z., Griffith, R., Hussain, F., Mu, D., Nie, Y., Chen, P., Zhou, W., & Ruan, R. (2014). Swine manure-based pilot-scale algal biomass production system for fuel production and wastewater treatment—a case study. Applied Biochemistry and Biotechnology, 172(3), 1390–1406. https://doi.org/10.1007/s12010-013-0603-6.
Sun, Z. L., Sun, L. Q., & Chen, G. Z. (2019). Microalgal cultivation and nutrient removal from digested piggery wastewater in a thin-film flat plate photobioreactor. Applied Biochemistry and Biotechnology, 187(4), 1488–1501. https://doi.org/10.1007/s12010-018-2889-x.
Zhou, W., Hu, B., Li, Y., Min, M., Mohr, M., Du, Z., Chen, P., & Ruan, R. (2012). Mass cultivation of microalgae on animal wastewater: a sequential two-stage cultivation process for energy crop and omega-3-rich animal feed production. Applied Biochemistry and Biotechnology, 168(2), 348–363. https://doi.org/10.1007/s12010-012-9779-4.
Carr, N. G., & Whitton, B. A. (1973). The biology of blue-green algae. Blackwell Scientific Publications.
Sharathchandra, K., & Rajashekhar, M. (2011). Total lipid and fatty acid composition in some freshwater cyanobacteria. Journal Algal Biomass Utilization, 2, 83–97.
Kushwaha, D., Upadhyay, S. N., & Mishra, P. K. (2017). Growth of cyanobacteria: optimization for increased carbohydrate content. Applied Biochemistry and Biotechnology, 184(4), 1247–1262. https://doi.org/10.1007/s12010-017-2620-3.
Kwietniewska, E., & Tys, J. (2014). Process characteristics, inhibition factors and methane yields of anaerobic digestion process, with particular focus on microalgal biomass fermentation. Renewable and Sustainable Energy Reviews, 34, 491–500. https://doi.org/10.1016/j.rser.2014.03.041.
Conforti, V. T., Ruiz, L. B., & Leonardi, P. I. (2017). Ultrastructural alterations in Lepocinclis acus (Euglenophyta) induced by medium with high organic matter content. Frontiers in Ecology and Evolution, 5, 1–8. https://doi.org/10.3389/fevo.2017.00141.
Gonzalez-Fernandez, C., Sialve, B., & Molinuevo-Salces, B. (2015). Anaerobic digestion of microalgal biomass: challenges, opportunities and research needs. Bioresource Technology, 198, 896–906. https://doi.org/10.1016/j.biortech.2015.09.095.
Kovačić, Đ., Kralik, D., Jovičić, D., Rupčić, S., Popović, B., & Tišma, M. (2018). Thermal pretreatment of harvest residues and their use in anaerobic codigestion with dairy cow manure. Applied Biochemistry and Biotechnology, 184(2), 471–483. https://doi.org/10.1007/s12010-017-2559-4.
Khalid, A., Arshad, M., Anjum, M., Mahmood, T., & Dawson, L. (2011). The anaerobic digestion of solid organic waste. Waste Management, 31(8), 1737–1744. https://doi.org/10.1016/j.wasman.2011.03.021.
Keramati, M., & Beiki, H. (2017). The effect of pH adjustment together with different substrate to inoculum ratios on biogas production from sugar beet wastes in an anaerobic digester. Journal of Energy Management Technololgy, 1, 6–11 https://doi.org/10.22109/jemt.2017.87623.1016.
Zhang, C., Su, H., Baeyens, J., & Tan, T. (2014). Reviewing the anaerobic digestion of food waste for biogas production. Renewable and Sustainable Energy Reviews, 38, 383–392. https://doi.org/10.1016/j.rser.2014.05.038.
Liu, C., Yuan, X., Zeng, G., Li, W., & Li, J. (2008). Prediction of methane yield at optimum pH for anaerobic digestion of organic fraction of municipal solid waste. Bioresource Technology, 99(4), 882–888. https://doi.org/10.1016/j.biortech.2007.01.013.
Yen, H. W., & Brune, D. E. (2007). Anaerobic codigestion of algal sludge and waste paper to produce methane. Bioresource Technology, 98(1), 130–134. https://doi.org/10.1016/j.biortech.2005.11.010.
Ward, A. J. D., Lewis, M., & Green, F. B. (2014). Anaerobic digestion of algae biomass: a review. Algal Research, 5, 204–214. https://doi.org/10.1016/j.algal.2014.02.001.
Uggetti, E., Passos, F., Solé, M., Garfí, M., & Ferrer, I. (2017). Recent achievements in the production of biogas from microalgae. Waste and Biomass Valorization, 8(1), 129–139. https://doi.org/10.1007/s12649-016-9604-3.
Dębowski, M., Zieliński, M., Kisielewska, M., & Krzemieniewski, M. (2017). Anaerobic codigestion of the energy crop Sida Hermaphrodita and microalgae biomass for enhanced biogas production. International Journal of Environmental Research, 11(3), 243–250. https://doi.org/10.1007/s41742-017-0024-4.
Sialve, B., Bernet, N., & Bernard, O. (2009). Anaerobic digestion of microalgae as a necessary step to make microalgal biodiesel sustainable. Biotechnology Advances, 27(4), 409–416. https://doi.org/10.1016/j.biotechadv.2009.03.001.
Thorin, E., Olsson, J., Schwede, S., & Nehrenheim, E. (2018). Codigestion of sewage sludge and microalgae – biogas production Investigations. Applied Energy, 227, 64–72. https://doi.org/10.1016/j.apenergy.2017.08.085.
Bohutskyi, P., Keller, T. A., Phan, D., Parris, M. L., Li, M., Richardson, L., & Kopachevsky, A. M. (2019). Codigestion of wastewater-grown filamentous algae with sewage sludge improves biomethane production and energy balance compared to thermal, chemical, or thermochemical pretreatments. Frontiers in Energy Research, 7, 47. https://doi.org/10.3389/fenrg.2019.00047.
Funding
This work was funded through institutional financial support from the Faculty of Agrobiotechnical Sciences and the Department of Biology, University of Josip Juraj Strossmayer in Osijek, Croatia.
Author information
Authors and Affiliations
Contributions
The concept and design of the study, analysis and interpretation of data, and manuscript writing were done by Deže, D., Mihaljević, M., Kralik, D., and Kovačić, Đ. supported by Jovičić, D. in experiment realization and calculations of the given results.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that there are no conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Deže, D., Mihaljević, M., Kovačić, Đ. et al. Natural Communities of Microalgae and Cyanobacteria from Eutrophicated Waters as Potential Co-substrates for Small-scale Biogas Production. Appl Biochem Biotechnol 192, 1016–1028 (2020). https://doi.org/10.1007/s12010-020-03382-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-020-03382-y