Spatio-Temporal Variability of Chlorophyll-A and Environmental Variables in the Panama Bight
Abstract
:1. Introduction
2. Materials and Methods
2.1. General Features of the Study Area
2.2. Surface Satellite Chlorophyll-a
2.3. Physical Environmental Drivers
2.4. River Discharges
2.5. Nutrient Content
2.6. Data Processing and Statistical Analysis
3. Results
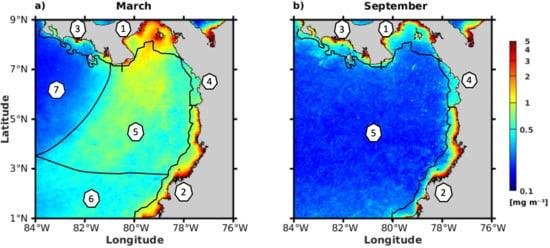
3.1. Spatio-Temporal Variability of the Annual Cycle of Chlorophyll-a
3.2. Spatial Characterization of the Environmental Variables in March and September
3.2.1. Wind Field and Ekman Pumping
3.2.2. Geostrophic Circulation Field and Sea Level Anomalies
3.2.3. Mixed Layer Depth
3.2.4. Photosynthetically Available Radiation
3.2.5. Sea Surface Temperature
3.3. Spatial Distribution of Nutrient Content in March and September
4. Discussion
4.1. Association between Chlorophyll-a and the Environmental Drivers
4.2. Characterization of Chlorophyll-a by Subdomains in Association with Physical Drivers and Nutrient Availability
4.3. Reliability of Satellite Data
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A


References
- Field, C.B. Primary Production of the Biosphere: Integrating Terrestrial and Oceanic Components. Science 1998, 281, 237–240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sigman, D.M.; Hain, M.P. The Biological Productivity of the Ocean. Nat. Educ. 2012, 3, 1–16. [Google Scholar]
- Falkowski, P.G.; Oliver, M.J. Mix and match: How climate selects phytoplankton. Nat. Rev. Microbiol. 2007, 5, 813–819. [Google Scholar] [CrossRef]
- Ji, R.; Edwards, M.; MacKas, D.L.; Runge, J.A.; Thomas, A.C. Marine plankton phenology and life history in a changing climate: Current research and future directions. J. Plankton Res. 2010, 32, 1355–1368. [Google Scholar] [CrossRef] [PubMed]
- Racault, M.F.; Le Quéré, C.; Buitenhuis, E.; Sathyendranath, S.; Platt, T. Phytoplankton phenology in the global ocean. Ecol. Indic. 2012, 14, 152–163. [Google Scholar] [CrossRef]
- Racault, M.F.; Platt, T.; Sathyendranath, S.; Aǧirbaş, E.; Martinez Vicente, V.; Brewin, R. Plankton indicators and ocean observing systems: Support to the marine ecosystem state assessment. J. Plankton Res. 2014, 36, 621–629. [Google Scholar] [CrossRef] [Green Version]
- Ward, B.A. Temperature-correlated changes in phytoplankton community structure are restricted to polar waters. PLoS ONE 2015, 10. [Google Scholar] [CrossRef] [Green Version]
- Corredor-Acosta, A.; Morales, C.E.; Hormazabal, S.; Andrade, I.; Correa-Ramirez, M.A. Phytoplankton phenology in the coastal upwelling region off central-southern Chile (35°S–38°S): Time-space variability, coupling to environmental factors, and sources of uncertainty in the estimates. J. Geophys. Res. Ocean. 2015, 120, 813–831. [Google Scholar] [CrossRef]
- Lévy, M.; Franks, P.J.; Smith, K.S. The role of submesoscale currents in structuring marine ecosystems. Nat. Commun. 2018, 9, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Salgado-Hernanz, P.M.; Racault, M.F.; Font-Muñoz, J.S.; Basterretxea, G. Trends in phytoplankton phenology in the Mediterranean Sea based on ocean-colour remote sensing. Remote Sens. Envion. 2019, 221, 50–64. [Google Scholar] [CrossRef]
- Pennington, J.T.; Mahoney, K.L.; Kuwahara, V.S.; Kolber, D.D.; Calienes, R.; Chavez, F.P. Primary production in the eastern tropical Pacific: A review. Prog. Oceanogr. 2006, 69, 285–317. [Google Scholar] [CrossRef]
- Chelton, D.B.; Freilich, M.H.; Esbensen, S.K. Satellite Observations of the Wind Jets off the Pacific Coast of Central America. Part I: Case Studies and Statistical Characteristics. Mon. Weather Rev. 2000, 128, 1993–2018. [Google Scholar] [CrossRef]
- Amador, J.A.; Alfaro, E.J.; Lizano, O.G.; Magaña, V.O. Atmospheric forcing of the eastern tropical Pacific: A review. Prog. Oceanogr. 2006, 69, 101–142. [Google Scholar] [CrossRef]
- Marrari, M.; Piola, A.R.; Valla, D. Variability and 20-Year Trends in Satellite-Derived Surface Chlorophyll Concentrations in Large Marine Ecosystems around South and Western Central America. Front. Mar. Sci. 2017, 4, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Clarke, A.J. El Niño physics and El Niño predictability. Annu. Rev. Mar. Sci. 2014, 6, 79–99. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Weisberg, R.H. The 1997-98 El Niño evolution relative to previous El Niño events. J. Clim. 2000, 13, 488–501. [Google Scholar] [CrossRef]
- McPhaden, M.J.; Zebiak, S.E.; Glantz, M.H. ENSO as an integrating concept in earth science. Science 2006, 314, 1740–1745. [Google Scholar] [CrossRef] [Green Version]
- Gierach, M.M.; Lee, T.; Turk, D.; McPhaden, M.J. Biological response to the 1997–98 and 2009–10 El Niño events in the equatorial Pacific Ocean. Geophys. Res. Lett. 2012, 39. [Google Scholar] [CrossRef]
- Radenac, M.H.; Léger, F.; Singh, A.; Delcroix, T. Sea surface chlorophyll signature in the tropical Pacific during eastern and central Pacific ENSO events. J. Geophys. Res. Ocean. 2012, 117. [Google Scholar] [CrossRef] [Green Version]
- Messié, M.; Chavez, F.P. A global analysis of ENSO synchrony: The oceans’ biological response to physical forcing. J. Geophys. Res. Ocean. 2012, 117. [Google Scholar] [CrossRef]
- Racault, M.F.; Sathyendranath, S.; Menon, N.; Platt, T. Phenological responses to ENSO in the global oceans. Surv. Geophys. 2017, 38, 277–293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Christian, J.R.; Verschell, M.A.; Murtugudde, R.; Busalacchi, A.J.; McClain, C.R. Biogeochemical modelling of the tropical Pacific Ocean. I: Seasonal and interannual variability. Deep Sea Res. Part II Top. Stud. Oceanogr. 2001, 49, 509–543. [Google Scholar] [CrossRef]
- Radenac, M.H.; Menkès, C.; Vialard, J.; Moulin, C.; Dandonneau, Y.; Delcroix, T.; Dupouy, C.; Stoens, A.; Deschamps, P.-Y. Modeled and observed impacts of the 1997–1998 El Niño on nitrate and new production in the equatorial Pacific. J. Geophys. Res. Ocean. 2001, 106, 26879–26898. [Google Scholar] [CrossRef] [Green Version]
- Sharma, P.; Singh, A.; Marinov, I.; Kostadinov, T. Contrasting ENSO Types with Satellite-Derived Ocean Phytoplankton Biomass in the Tropical Pacific. Geophys. Res. Lett. 2019, 46, 5987–5996. [Google Scholar] [CrossRef] [Green Version]
- Messié, M.; Radenac, M.H. Seasonal variability of the surface chlorophyll in the western tropical Pacific from SeaWiFS data. Deep Sea Res. Part I Oceanogr. Res. Pap. 2006, 53, 1581–1600. [Google Scholar] [CrossRef]
- Romero-Torres, M.; Acosta, A.; Palacio-Castro, A.M.; Treml, E.A.; Zapata, F.A.; Paz-García, D.A.; Porter, J.W. Coral reef resilience to thermal stress in the Eastern Tropical Pacific. Glob. Chang. Biol. 2020, 26, 3880–3890. [Google Scholar] [CrossRef]
- Rivera-Gómez, M.; Giraldo, A.; Lavaniegos, B.E. Structure of euphausiid assemblages in the Eastern Tropical Pacific off Colombia during El Niño, La Niña and Neutral conditions. J. Exp. Mar. Biol. Ecol. 2019, 516, 1–15. [Google Scholar] [CrossRef]
- Wooster, W.S. Oceanographic Observations in the Panama Bight, “Askoy” Expedition, 1941. Bull. Am. Mus. Nat. Hist. 1959, 118, 113–152. [Google Scholar]
- Spalding, M.D.; Fox, H.E.; Allen, G.R.; Davidson, N.; Ferdaña, Z.A.; Finlayson, M.; Halpern, B.S.; Jorge, M.A.; Lombana, A.; Lourie, S.A.; et al. Marine Ecoregions of the World: A Bioregionalization of Coastal and Shelf Areas. Bioscience 2007, 57, 573–583. [Google Scholar] [CrossRef] [Green Version]
- Villegas Bolaños, N.L.; Málikov, I.; Díaz, D. Variabilidad mensual de la velocidad de surgencia y clorofila a en la región del Panama Bight. Rev. Mutis. 2016, 6, 82–94. [Google Scholar] [CrossRef] [Green Version]
- CCCP. Compilación Oceanográfica de la Cuenca Pacífica Colombiana; Centro Control Contaminación del Pacífico, DIMAR: Cartagena, Colombia, 2002; pp. 1–124. [Google Scholar]
- Rodríguez Rubio, E.; Stuardo, J. Variability of photosynthetic pigments in the Colombian Pacific Ocean and its relationship with the wind field using ADEOS-I data. J. Earth Syst. Sci. 2002, 111, 227–236. [Google Scholar] [CrossRef] [Green Version]
- Poveda, G.; Mesa, O.J. La corriente de Chorro Superficial del Oeste (“del Chocó”) y otras dos corrientes de Chorro en Colombia: Climatología y variabilidad durante fases del ENSO. Rev. Acad. Colomb. Cienc. 1999, 23, 517–528. [Google Scholar]
- Rodríguez-Rubio, E.; Schneider, W.; Abarca del Río, R. On the seasonal circulation within the Panama Bight derived from satellite observations of wind, altimetry and sea surface temperature. Geophys. Res. Lett. 2003, 30, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Willett, C.S.; Leben, R.R.; Lavín, M.F. Eddies and Tropical Instability Waves in the eastern tropical Pacific: A review. Prog. Oceanogr. 2006, 69, 218–238. [Google Scholar] [CrossRef]
- Devis-Morales, A.; Schneider, W.; Montoya-Sánchez, R.A.; Rodríguez Rubio, E. Monsoon-like winds reverse oceanic circulation in the Panama Bight. Geophys. Res. Lett. 2008, 35, 1–6. [Google Scholar] [CrossRef]
- D’Croz, L.; O’Dea, A. Variability in upwelling along the Pacific shelf of Panama and implications for the distribution of nutrients and chlorophyll. Estuar. Coast. Shelf Sci. 2007, 73, 325–340. [Google Scholar] [CrossRef]
- Poveda, G.; Mesa, O.J. On the Existence of Lloró (the Rainiest Locality on Earth): Enhanced Ocean-Land-Atmosphere Interaction by a Low-Level Jet. Geophys. Res. Lett. 2000, 27, 1675–1678. [Google Scholar] [CrossRef] [Green Version]
- CONAGUA. 2016. Available online: http://www.conagua.gob.pa/pnsh/estado-del-agua/el-agua-en-panama.html (accessed on 29 March 2019).
- DeMaster, D.J. The Global Marine Silica Budget: Sources and Sinks. In Encyclopedia of Ocean Sciences, 3rd ed.; American Press: London, UK, 2018; pp. 1–655. [Google Scholar] [CrossRef]
- Tyrrell, T. Redfield Ratio. In Encyclopedia of Ocean Sciences, 3rd ed.; American Press: London, UK, 2018; pp. 461–472. [Google Scholar] [CrossRef]
- Lonsdale, P. Inflow of bottom water to the Panama Basin. Deep Sea Res. 1977, 24, 1065–1101. [Google Scholar] [CrossRef]
- Tchantsev, V.; Cabrera-Luna, E. Algunos aspectos de investigación de la formación del régimen oceanográfico en el Pacífico colombiano. Boletín Científico CCCP 1998, 7–19. [Google Scholar] [CrossRef]
- Sathyendranath, S.; Brewin, R.J.; Brockmann, C.; Brotas, V.; Calton, B.; Chuprin, A.; Cipollini, P.; Couto, A.B.; Dingle, J.; Doerffer, R.; et al. An Ocean-Colour Time Series for Use in Climate Studies: The Experience of the Ocean-Colour Climate Change Initiative (OC-CCI). Sensors 2019, 19, 4285. [Google Scholar] [CrossRef] [Green Version]
- Kessler, W.S. Mean three-dimensional circulation in the northeast tropical Pacific. J. Phys. Oceanogr. 2002, 32, 2457–2471. [Google Scholar] [CrossRef] [Green Version]
- Holte, J.; Talley, L. A new algorithm for finding mixed layer depths with applications to argo data and subantarctic mode water formation. J. Atmos. Ocean. Technol. 2009, 26, 1920–1939. [Google Scholar] [CrossRef]
- Johnson, G.C.; Schmidtko, S.; Lyman, J.M. Relative contributions of temperature and salinity to seasonal mixed layer density changes and horizontal density gradients. J. Geophys. Res. Ocean. 2012, 117, 1–13. [Google Scholar] [CrossRef]
- Schmidtko, S.; Johnson, G.C.; Lyman, J.M. MIMOC: A global monthly isopycnal upper-ocean climatology with mixed layers. J. Geophys. Res. Ocean. 2013, 118, 1658–1672. [Google Scholar] [CrossRef] [Green Version]
- ETESA. 2019. Available online: https://www.hidromet.com.pa/open_data.php (accessed on 1 August 2019).
- IDEAM. 2019. Available online: http://www.ideam.gov.co/solicitud-de-informacion (accessed on 1 September 2019).
- Navarra, A.; Simoncini, V. A guide to Empirical Orthogonal Functions for Climate Data Analysis; Springer: London, UK, 2010; pp. 107–121. [Google Scholar] [CrossRef]
- Garcia, C.A.; Garcia, V.M. Variability of chlorophyll-a from ocean color images in the La Plata continental shelf region. Cont. Shelf Res. 2008, 28, 1568–1578. [Google Scholar] [CrossRef]
- Domínguez-Hernández, G.; Cepeda-Morales, J.; Soto-Mardones, L.; Rivera-Caicedo, J.P.; Romero-Rodríguez, D.A.; Inda-Díaz, E.A.; Hernández-Almeida, O.U.; Romero-Bañuelos, C. Semi-annual variations of chlorophyll concentration on the Eastern Tropical Pacific coast of Mexico. Adv. Space Res. 2020, 65, 2595–2607. [Google Scholar] [CrossRef]
- Anderson, M.; Gorley, R.N.; Clarke, K.R. PERMANOVA+ for PRIMER. Guide to Software and Statistical Methods; PRIMER-E: Plymouth, UK, 2008. [Google Scholar]
- Akaike, H. Information theory as an extension of the maximum likelihood principle. In International Symposium on Information Theory, 2nd ed.; Petrov, B., Caski, F., Eds.; Akademiai Kiado: Budapest, Hungary, 1973; pp. 267–281. [Google Scholar]
- Clarke, K.R.; Gorley, R.N. PRIMER v6: User Manual/Tutorial; PRIMER-E: Plymouth, UK, 2006. [Google Scholar]
- Minchin, P.R. An evaluation of the relative robustness of techniques for ecological ordination. In Theory and Models in Vegetation Science. Advances in Vegetation Science; Prentice, I.C., van der Maarel, E., Eds.; Springer: Dordrecht, The Netherlands, 1987; pp. 89–107. [Google Scholar]
- Legendre, P.; Legendre, L. Numerical Ecology, 3rd ed.; Elsevier: Amsterdam/Dordrecht, The Netherlands, 2012; pp. 512–519. [Google Scholar]
- McGillicuddy, D., Jr. Mechanisms of physical-biological-biogeochemical interaction at the oceanic mesoscale. Annu. Rev. Mar. Sci. 2016, 8, 125–159. [Google Scholar] [CrossRef] [Green Version]
- Rueda, Ó.; Poveda, G. Variabilidad espacial y temporal del Chorro del Chocó y su efecto en la hidroclimatología de la región del Pacífico colombiano. Meteorol. Colomb. 2006, 10, 132–145. [Google Scholar]
- Ramírez, D.G.; Giraldo, A.; Tovar, J. Producción primaria, biomasa y composición taxonómica del fitoplancton costero y oceánico en el Pacífico colombiano (septiembre-octubre 2004). Investig. Mar. 2006, 34, 211–216. [Google Scholar] [CrossRef] [Green Version]
- Sogamoso, E.A.; Rubio, E.R.; Galeano, A.M. Distribución, abundancia y composición del fitoplancton y condiciones ambientales en la cuenca Pacífica colombiana, durante enero-febrero de 2007. Boletín Científico CCCP 2008, 15, 105–122. [Google Scholar] [CrossRef] [Green Version]
- INVEMAR-ANH. Especies, Ensamblajes y Paisajes de los Bloques Marinos Sujetos a Exploración de Hidrocarburos; Informe de actividades INVEMAR-ANH Fase III-Pacífico Caracterización de la megafauna y el plancton del Pacífico Colombiano: Santa Marta, Colombia, 2010; p. 226. [Google Scholar]
- Castillo, F.A.; Vizcaíno, Z. Los indicadores biológicos del fitoplancton y su relación con el fenómeno de El Niño 1991-92 en el Pacífico colombiano. Bol. Cient. Cioh. 1992, 13–22. [Google Scholar] [CrossRef]
- Montagnes, D.J.; Franklin, M. Effect of temperature on diatom volume, growth rate, and carbon and nitrogen content: Reconsidering some paradigms. Limnol. Oceanogr. 2001, 46, 2008–2018. [Google Scholar] [CrossRef] [Green Version]
- Atkinson, D.; Ciotti, B.J.; Montagnes, D.J. Protists decrease in size linearly with temperature: Ca. 2.5% C−1. Proc. R. Soc. Lond. (Boil.) 2003, 270, 2605–2611. [Google Scholar] [CrossRef] [Green Version]
- Marañón, E.; Steele, J.; Thorpe, A.; Turekian, K. Phytoplankton size structure. In Elements of Physical Oceanography: A Derivative of the Encyclopedia of Ocean Sciences, 2nd ed.; Academic Press: Washington, WA, USA, 2009; pp. 1–85. [Google Scholar]
- Marañón, E.; Lorenzo, M.P.; Cermeño, P.; Mouriño-Carballido, B. Nutrient limitation suppresses the temperature dependence of phytoplankton metabolic rates. ISME J. 2018, 12, 1836–1845. [Google Scholar] [CrossRef] [Green Version]
- Kruskopf, M.; Flynn, K.J. Chlorophyll content and fluorescence responses cannot be used to gauge reliably phytoplankton biomass, nutrient status or growth rate. New Phytol. 2006, 169, 525–536. [Google Scholar] [CrossRef]
- Bellacicco, M.; Volpe, G.; Colella, S.; Pitarch, J.; Santoleri, R. Influence of photoacclimation on the phytoplankton seasonal cycle in the Mediterranean Sea as seen by satellite. Remote Sens. Environ. 2016, 184, 595–604. [Google Scholar] [CrossRef]
- Halsey, K.H.; Jones, B.M. Phytoplankton strategies for photosynthetic energy allocation. Annu. Rev. Mar. Sci. 2015, 7, 265–297. [Google Scholar] [CrossRef]
- Behrenfeld, M.J.; Boss, E.; Siegel, D.A.; Shea, D.M. Carbon-based ocean productivity and phytoplankton physiology from space. Glob. Biogeochem. Cycles 2005, 19, GB1006. [Google Scholar] [CrossRef]
- Hopkinson, B.M.; Barbeau, K.A. Interactive influences of iron and light limitation on phytoplankton at subsurface chlorophyll maxima in the eastern North Pacific. Limnol. Oceanogr. 2008, 53, 1303–1318. [Google Scholar] [CrossRef] [Green Version]
- Mahadevan, A. The impact of submesoscale physics on primary productivity of plankton. Annu. Rev. Mar. Sci. 2016, 8, 161–184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Atkinson, D. Temperature and organism size: A biological law for ectotherms? Adv. Ecol. Res. 1994, 25, 1–58. [Google Scholar] [CrossRef]
- Woods, H.A. Egg-mass size and cell size: Effects of temperature on oxygen distribution. Am. Zool. 1999, 39, 244–252. [Google Scholar] [CrossRef]
- Milliman, J.D.; Farnsworth, K.L. River Discharge to the Coastal Ocean. A Global Synthesis, 1st ed.; Cambridge University Press: Cambridge, UK, 2011; pp. 1–384. [Google Scholar]
- Restrepo, J. Aporte de caudales de los ríos Baudó, San Juan, Patía y Mira a la cuenca Pacífica colombiana. Boletín Científico CCCP 2006, 13, 17–32. [Google Scholar] [CrossRef]
- Restrepo, J.D.; Kjerfve, B. Environmental Geochemistry in Tropical and Subtropical Environments. Environmental Science. In The Pacific and Caribbean Rivers of Colombia: Water Discharge, Sediment Transport and Dissolved Loads; Drude de Lacerda, L., Santelli, R.E., Duursma, E.K., Abrão, J.J., Eds.; Springer: Berlin/Heidelberg, Germany, 2004; pp. 169–187. [Google Scholar] [CrossRef]
- Aurin, D.; Mannino, A.; Franz, B. Spatially resolving ocean color and sediment dispersion in river plumes, coastal systems, and continental shelf waters. Remote Sens. Environ. 2013, 137, 212–225. [Google Scholar] [CrossRef] [Green Version]
- Saldías, G.S.; Largier, J.L.; Mendes, R.; Pérez-Santos, I.; Vargas, C.A.; Sobarzo, M. Satellite-measured interannual variability of turbid river plumes off central-southern Chile: Spatial patterns and the influence of climate variability. Prog. Oceanogr. 2016, 146, 212–222. [Google Scholar] [CrossRef]
- Behrenfeld, M.J.; Westberry, T.; Boss, E.; O’Malley, R.; Siegel, D.; Wiggert, J.D.; Franz, B.; Feldman, G.; Doney, S.; Moore, J.; et al. Satellite-Detected Fluorescence Reveals Global Physiology of Ocean Phytoplankton. Biogeosciences 2009, 6, 779–794. [Google Scholar] [CrossRef] [Green Version]
- Yokomizo, H.; Botsford, L.W.; Holland, M.D.; Lawrence, C.A.; Hastings, A. Optimal wind patterns for biological production in shelf ecosystems driven by coastal upwelling. Theor. Ecol. 2010, 3, 53–63. [Google Scholar] [CrossRef] [Green Version]
- Miloslavich, P.; Klein, E.; Díaz, J.M.; Hernandez, C.E.; Bigatti, G.; Campos, L.; Artigas, F.; Castillo, J.; Penschaszadeh, P.E.; Neill, P.E.; et al. Marine biodiversity in the Atlantic and Pacific coasts of South America: Knowledge and gaps. PLoS ONE 2011, 6, e14631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zapata, F.A.; Ross Robertson, D. How many species of shore fishes are there in the Tropical Eastern Pacific? J. Biogeogr. 2007, 34, 38–51. [Google Scholar] [CrossRef]
- Brewin, R.J.; Sathyendranath, S.; Jackson, T.; Barlow, R.; Brotas, V.; Airs, R.; Lamont, T. Influence of light in the mixed-layer on the parameters of a three-component model of phytoplankton size class. Remote Sens. Envion. 2015, 168, 437–450. [Google Scholar] [CrossRef]
- Brewin, R.J.; Ciavatta, S.; Sathyendranath, S.; Jackson, T.; Tilstone, G.; Curran, K.; Airs, R.L.; Cummings, D.; Brotas, V.; Organelli, E.; et al. Uncertainty in ocean-color estimates of chlorophyll for phytoplankton groups. Front. Mar. Sci. 2017, 4, 104. [Google Scholar] [CrossRef] [Green Version]
- Corredor-Acosta, A.; Morales, C.E.; Brewin, R.J.; Auger, P.A.; Pizarro, O.; Hormazabal, S.; Anabalón, V. Phytoplankton size structure in association with mesoscale eddies off central-southern Chile: The satellite application of a phytoplankton size-class model. Remote Sens. 2018, 10, 834. [Google Scholar] [CrossRef] [Green Version]
- Atlas, R.; Hoffman, R.N.; Ardizzone, J.; Leidner, S.M.; Jusem, J.C.; Smith, D.K.; Gombos, D. A cross-calibrated, multiplatform ocean surface wind velocity product for meteorological and oceanographic applications. Bull. Am. Meteor. Soc. 2011, 92, 157–174. [Google Scholar] [CrossRef]
- ECMWF Copernicus, Product Quality Assessment Report, Sea Level. Available online: http://datastore.copernicus-climate.eu/documents/satellite-sea-level/D2.SL.2-v1.1_PQAR_of_v1DT2018_SeaLevel_products_v2.2.pdf (accessed on 19 June 2020).
- Chin, T.M.; Vazquez-Cuervo, J.; Armstrong, E.M. A multi-scale high-resolution analysis of global sea surface temperature. Remote Sens. Environ. 2017, 200, 154–169. [Google Scholar] [CrossRef]












| March | Variable | SS(trace) | Pseudo-F | P | % Explained |
| SST | 18.06 | 992.03 | 0.001 | 60.82 | |
| SLA | 1.59 | 117.87 | 0.001 | 5.35 | |
| GC | 1.44 | 90.68 | 0.001 | 4.87 | |
| Wind | 0.53 | 42.01 | 0.001 | 1.79 | |
| Best Solution | |||||
| AICc | R2 | RSS | No. Vars | Predictors | |
| −2794.8 | 0.7284 | 8.0623 | 4 | SST, GC, SLA, Wind | |
| September | Variable | SS(trace) | Pseudo-F | P | % Explained |
| Wind | 6.13 | 300.94 | 0.001 | 32.05 | |
| PAR | 4.25 | 310.00 | 0.001 | 22.24 | |
| EP | 0.81 | 68.84 | 0.001 | 4.27 | |
| SLA | 0.37 | 28.59 | 0.001 | 1.96 | |
| GC | 0.27 | 23.96 | 0.001 | 1.43 | |
| SST | 0.09 | 8.15 | 0.005 | 0.48 | |
| Best Solution | |||||
| AICc | R2 | RSS | No. Vars | Predictors | |
| −2859.4 | 0.6246 | 7.1809 | 6 | All | |
| Month | Subdomain | Mean Chl-a | n | Physical Variables | Nutrients | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SST | Wind | EP | GC | SLA | PAR | N | P | Si | Fe | ||||
| March | 1 | 1.14 | 28 | -- | -- | 0.42 | -0.43 | -- | -- | -- | -- | -- | 0.44 |
| 2 | 1.07 | 26 | 0.51 | -- | -- | 0.52 | -- | -- | -- | -- | -- | -- | |
| 3 | 0.57 | 26 | 0.48 | -- | -- | -- | -- | -- | 0.87 | 0.87 | 0.91 | 0.90 | |
| 5 | 0.79 | 318 | 0.72 | 0.73 | 0.12 | −0.22 | −0.55 | 0.23 | 0.58 | 0.64 | 0.11 | -- | |
| 6 | 0.66 | 129 | -- | -- | 0.51 | −0.39 | −0.49 | −0.46 | −0.47 | −0.49 | 0.41 | 0.24 | |
| 7 | 0.41 | 117 | −0.95 | 0.93 | −0.74 | 0.44 | −0.66 | 0.57 | 0.92 | 0.92 | 0.94 | 0.93 | |
| September | 1 | 0.67 | 28 | -- | 0.57 | -- | -- | -- | -- | -- | -- | -- | -- |
| 2 | 0.76 | 25 | 0.64 | −0.79 | 0.42 | -- | -- | -- | -- | -- | -- | -- | |
| 3 | 0.67 | 22 | 0.64 | −0.57 | -- | 0.51 | -- | 0.51 | -- | -- | -- | -- | |
| 5 | 0.33 | 565 | 0.27 | −0.50 | −0.11 | 0.25 | 0.12 | -- | −0.35 | −0.42 | 0.69 | 0.25 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Corredor-Acosta, A.; Cortés-Chong, N.; Acosta, A.; Pizarro-Koch, M.; Vargas, A.; Medellín-Mora, J.; Saldías, G.S.; Echeverry-Guerra, V.; Gutiérrez-Fuentes, J.; Betancur-Turizo, S. Spatio-Temporal Variability of Chlorophyll-A and Environmental Variables in the Panama Bight. Remote Sens. 2020, 12, 2150. https://doi.org/10.3390/rs12132150
Corredor-Acosta A, Cortés-Chong N, Acosta A, Pizarro-Koch M, Vargas A, Medellín-Mora J, Saldías GS, Echeverry-Guerra V, Gutiérrez-Fuentes J, Betancur-Turizo S. Spatio-Temporal Variability of Chlorophyll-A and Environmental Variables in the Panama Bight. Remote Sensing. 2020; 12(13):2150. https://doi.org/10.3390/rs12132150
Chicago/Turabian StyleCorredor-Acosta, Andrea, Náyade Cortés-Chong, Alberto Acosta, Matias Pizarro-Koch, Andrés Vargas, Johanna Medellín-Mora, Gonzalo S. Saldías, Valentina Echeverry-Guerra, Jairo Gutiérrez-Fuentes, and Stella Betancur-Turizo. 2020. "Spatio-Temporal Variability of Chlorophyll-A and Environmental Variables in the Panama Bight" Remote Sensing 12, no. 13: 2150. https://doi.org/10.3390/rs12132150