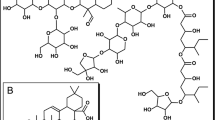
Abstract
Since the beginning, natural products have represented an important source of bioactive molecules for cancer treatment. Among them, cardenolides attract the attention of different research groups due to their cardiotonic and antitumor activity. The observed biological activity is closely related to their Na+/K+-ATPase inhibition potency. Currently, the discovery of new compounds against cancer is an urgent need in modern pharmaceutical research. Thus, the aim of this work is to determine the physicochemical properties and substituent effects that module the antiproliferative activity of cardenolides on the human lung cancer cell line A549. We build and curate a library with results obtained from literature; molecular descriptors were calculated in PaDEL software, and SAR/QSAR analysis was performed. The SAR results showed that cardenolides were sensitive to modifications in C and D steroidal ring and required substituent groups with the function of hydrogen bond acceptor at the C3 position. QSAR models to doubly linked-type cardenolides indicated that properties as lipoaffinity and atoms with the capacity to be hydrogen bond acceptors are involved in the increment of antiproliferative activity on A549 cell line. In contrast, the presence and position of very electro-negative atoms on the molecule decreased the antiproliferative effect on A549 cells. These results suggest that the antiproliferative capacity of cardenolides on the cell line A549 is strongly related to substituent groups on the C3 position, which must not be carbohydrate. Additionally, the steroidal rings C and D must remain without modifications.
Graphic abstract





Similar content being viewed by others
References
Bray F, Ferlay J, Soerjomataram I, Siegel R, Torre L, Jemal A (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Cancer J Clin 2018:1–31. https://doi.org/10.3322/caac.21492
World Health Organization (WHO) Mexico cancer statistics. Global Cancer Observatory GLOBOCAN. 2019. https://gco.iarc.fr/today/data/factsheets/populations/484-mexico-fact-sheets.pdf. Accessed July 2019
Ferlay J, Colombet M, Soerjomataram I, Mathers C, Parkin DM, Piñeros M, Znaor A, Bray F (2019) Estimating the global cancer incidence and mortality in 2018: GLOBOCAN source and methods. Int J Cancer 144:1941–1953. https://doi.org/10.1002/ijc.31937
Townsend MH, Anderson MD, Weagel EG, Velazquez EJ, Weber KS, Robison RA, O’Neill KL (2017) Non-small-cell lung cancer cell lines A549, and NCI-H460 express hypoxanthine-guanine phosphoribosyltransferase on the plasma membrane. Onco Targets Ther 10:1921–1932. https://doi.org/10.2147/ott.s128416
Harvey A (2000) Strategies for discovering drugs from previously unexplored natural products. Drug Discov Today 5(7):294–300. https://doi.org/10.1016/S1359-6446(00)01511-7
Palhares RM, Drummond MG, Brasil BDSAF, Cosenza GP, Brandão MDGL, Oliveira G (2015) Medicinal plants recommended by the world health organization: DNA barcode identification associated with chemical analyses guarantees their quality. PLoS ONE 10(5):1–29. https://doi.org/10.1371/journal.pone.0127866
Zeino M, Brenk R, Gruber L, Zhel M, Urban E, Brigitte K, Thomas E (2015) Cytotoxicity of cardiotonic steroids in sensitive and multidrug-resistant leukemia cells and the link with Na, K-ATPase. J Steroid Biochem Mol Biol 150:97–111. https://doi.org/10.1016/j.jsbmb.2015.03.008
Newman RA, Yang P, Pawlus AD, Block KI (2008) Cardiac glycosides as novel cancer therapeutic agents. Mol Interv 8(1):36–49. https://doi.org/10.1124/mi.8.1.8
Prassas I, Diamandis EP (2008) Novel therapeutic applications of cardiac glycosides. Nat Rev Drug Discov 7(11):926–935. https://doi.org/10.1038/nrd2682
Pierre SV, Xie Z (2006) The Na, K-ATPase receptor complex. Cell Biochem Biophys 46:303–315. https://doi.org/10.1385/CBB:46:3:303
Jorgensen PL, Håkansson KO, Karlish SJD (2003) Structure and mechanism of Na, K-ATPase: functional sites and their interactions. Annu Rev Physiol 65(1):817–849. https://doi.org/10.1146/annurev.physiol.65.092101.142558
Chiang-Chan E, Kuin-Wong S, Tuck-Chan H (2016) Apocynaceae species with antiproliferative and/or antiplasmodial properties: a review of ten genera. J Integr Med 14(4):269–284. https://doi.org/10.1016/S2095-4964(16)60261-3
Yang CW, Chang HY, Hsu HY, Lee YZ, Chang HS, Chen IS, Lee SJ (2017) Identification of anti-viral activity of the cardenolides, Na+/K+-ATPase inhibitors, against porcine transmissible gastroenteritis virus. Toxicol Appl Pharmacol 332:129–137. https://doi.org/10.1016/j.taap.2017.04.017
Kamtcha DW, Tene M, Bedane KG, Knauer L, Strohmann C, Tane P, Kusari S, Spiteller M (2018) Cardenolides from the stem bark of Salacia staudtiana. Fitoterapia 127:402–409. https://doi.org/10.1016/j.fitote.2018.04.008
Ihenetu K, Espinosa R, de Leon R, Planas G, Perez-Pinero A, Waldbeser L (2008) Digoxin and digoxin-like immunoreactive factors (DLIF) modulate the release of pro-inflammatory cytokines. Inflamm Res 57:519–523. https://doi.org/10.1007/s00011-008-7249-9
Wang JKT, Portbury S, Thomas MB, Barney S, Ricca DJ, Morris DL, Warner DS, Lo DC (2006) Cardiac glycosides provide neuroprotection against ischemic stroke: discovery by a brain slice-based compound screening platform. PNAS 103(27):10461–10466. https://doi.org/10.1073/pnas.0600930103
Datte JY, Ziegler A (2001) Pharmacological investigation on nigrescigenin cardenolide from Paquetina nigrescens (Afzel.) Bullock: comparative studies on cardiotonic effects of Paquetina nigrescens, g-strophanthin and noradrenaline in guinea-pig isolated atria. J Pharm Pharmacol 53:859–866. https://doi.org/10.1211/0022357011776018
Mijatovic T, Dufrasne F, Kiss R (2012) Cardiotonic steroids-mediated targeting of the Na+, K+-ATPase to combat chemoresistant cancers. Curr Med Chem 19(5):627–646. https://doi.org/10.1055/s-0032-1328243
Calderón-Montaño JM, Burgos-Morón E, López-Lázaro M (2014) The in vivo antitumor activity of cardiac glycosides in mice xenografted with human cancer cells is probably an experimental artifact. Oncogene 33(22):2947–2948. https://doi.org/10.1038/onc.2013.229
Krishna AB, Manikyam HM, Sharma VK, Sharma N (2015) Plant cardenolides in therapeutics. Int J Indig Med Plants 48(2):1871–1896
Wen S, Chen Y, Lu Y, Wang Y, Diang L, Jiang M (2016) Cardenolides from Apocynaceae and their anticancer activity. Fitoterapia 112:74–84. https://doi.org/10.1016/j.fitote.2016.04.023
Rascón-Valenzuela LA, Velázquez-Contreras CA, Garibay-Escobar A, Medina-Juárez LA, Vilegas W, Robles-Zepeda RE (2015) Antiproliferative activity of cardenolide glycosides from Asclepias subulata. J Ethnopharmacol 171:280–286. https://doi.org/10.1016/j.jep.2015.05.057
Lu ZJ, Zhou Y, Song Q, Qin Z, Hong Z, Yong J, Gou TL, Yang JL, Lou F (2010) Periplocin inhibits the growth of lung cancer in vitro and in vivo by blocking AKT/ERK signaling pathways. Cell Physiol Biochem 26:609–618. https://doi.org/10.1159/000322328
Van Quaquebeke E, Simon G, Dewelle J, El Yazidi M, Braekman J, Kiss R et al (2005) Identification of a novel cardenolide (2′′-Oxovoruscharin) from Calotropis procera and the hemisynthesis of novel derivatives displaying potent in vitro antitumor activities and high in vivo tolerance: structure-activity relationship analyses. J Med Chem 48:849–856. https://doi.org/10.1021/jm049405a
Prieto-Martinez FD, Lopez-Lopez E, Juarez-Mercado KE, Medina-Franco JL (2019) Computational drug design methods—current and future perspectives. Chapter II. Silico Drug Des 2019:19–44. https://doi.org/10.1016/b978-0-12-816125-8.00002-x
Vujasinović I, Paravić-Radičević A, Mlinarić-Majerski K, Brajša K, Bertoša B (2012) Synthesis and biological validation of novel pyrazole derivatives with anticancer activity guided by 3D-QSAR analysis. Bioorganic Med Chem 20(6):2101–2110. https://doi.org/10.1016/j.bmc.2012.01.032
Speck-Planke A, Kleandrova VV, Luan F, Cordeiro MN (2012) Chemoinformatics in anti-cancer chemotheraphy: multi-target QSAR model for the silico discovery of anti-breast cancer agents. Eur J Pharm Sci 47:273–279. https://doi.org/10.1016/j.ejps.2012.04.012
Joy A, Alam A (2012) Quantitative structure-activity relationship (QSAR) of cardiac glycosides: the development of predictive in vitro cytotoxic activity model. Der Pharm Lett 4(4):1246–1269
Quadri L, Cerri A, Ferrari P, Folpini E, Mabilia M, Melloni P (1996) Synthesis of quantitative structure-activity relationship of 17β-(Hydrazonomethyl)-5β-androstane-3β-14β-diol derivates that bind to Na/K-ATPase receptor. J Med Chem 39:3385–3393. https://doi.org/10.1021/jm950806n
Cerri A, Serra F, Ferrari P, Folpini E, Padoani G, Melloni P (1997) Synthesis, cardiotonic activity, and structure–activity relationship of 17β-guanylhydrazone derivates of 5β-androstane-3β-14β-diol acting of the Na, K-ATPase receptor. J Med Chem 40:3484–3488. https://doi.org/10.1021/jm970312l
Gupta SP (2000) Quantitative structure–activity relationship of cardiotonic agents. Prog Drug Res 2000:235–282. https://doi.org/10.1007/978-3-0348-8385-6_7
Kamano Y, Yamashita A, Nogawa T, Morita H, Takeya K, Itokawa H, Segawa T, Yukita A, Saito K, Katsuyama M, Pettit GR (2002) QSAR evaluation of the Ch’an Su and related bufadienolides against the colchicine-resistant primary liver carcinoma cell line PLC/PRF/5. J Med Chem 45(25):5440–5447. https://doi.org/10.1021/jm0202066
Roy MC, Chang F, Huang H, Chiang MY, Wu Y (2005) Cytotoxic principles from the formosan milkweed, Asclepias curassavica. J Nat Prod 68:1494–1499. https://doi.org/10.1021/np0501740
Pan Z, Li Y, Liu J, Ning D, Li D (2012) A cytotoxic cardenolide and saponin from the rhizomes of Tupistra chinensis. Fitoterapia 83(8):1489–1493. https://doi.org/10.1016/j.fitote.2012.08.015
Xue R, Han N, Ye C, Wang L, Yang J, Wang Y (2014) The cytotoxic activities of cardiac glycosides from Streptocaulon juventas and the structure-activity relationships. Fitoterapia 98:228–233. https://doi.org/10.1016/j.fitote.2014.08.008
Mohamed NH, Liu M, Abdel-Mageed WM, Alwahibi LH, Dai H, Ahmed M, Badr G, Quinn RJ, Liu X, Zhang L, Shoreit AAM (2015) Cytotoxic cardenolides from the latex of Calotropis procera. Bioorg Med Chem Lett 25(20):4615–4620. https://doi.org/10.1016/j.bmcl.2015.08.044
Schneider NFZ, Cerella C, Lee JY, Mazumder A, Kim KR, de Carvalho A, Munkert J, Padua RM, Kreis W, Kim KW, Christov C, Dicato M, Kim HJ, Han BW, Braga FC, Simoes CMO, Diederich M (2018) Cardiac glycoside glucoevatromonoside induces cancer type-specific cell death. Front Pharmacol 9(3):1–17. https://doi.org/10.3389/fphar.2018.00070
Nolte E, Sobel A, Wach S, Hertlein H, Ebert N, Müller-Uri F, Slany R, Taubert H, Wullich B, Kreis W (2015) The new semisynthetic cardenolide analog 3β-[2-(1-Amantadine)-1-on-ethylamine]-digitoxigenin (AMANTADIG) efficiently suppresses cell growth in human leukemia and urological tumor cell lines. Anticancer Res 35(10):5271–5276
Heins M, Wahl J, Lerch H, Kaiser F, Reinhard E (1978) Preparation of beta-methyldigoxin by hydroxylation of beta-methyldigitoxin in fermenter cultures of Digitalis lanata. Planta Med 33(1):57–62. https://doi.org/10.1055/s-0028-1097359
Reinhard E, Kreis W, Barthlen U, Helmbold U (1989) Semicontinuous cultivation of Digitalis lanata cells: production of β-methyldigoxin in a 300-L airlift bioreactor. Biotechnol Bioeng 34(4):502–508. https://doi.org/10.1002/bit.260340410
Sawlewicz L, Linde HHA, Meyer K (1970) 162. 3-O-phosphoryl-digitoxigenin. Helv Chim Acta 53(161):1382–1385. https://doi.org/10.1002/hlca.19700530618
Bravo-Gómez ME, García-Ramos JC, Gracia-Mora I, Ruiz-Azuara L (2009) Antiproliferative activity and QSAR study of copper(II) mixed chelate [Cu(N-N)(acetylacetonate)]NO3 and [Cu(N-N)(glycinate)]NO3 complexes, (Casiopeínas®). J Inorg Biochem 103(2):299–309. https://doi.org/10.1016/j.jinorgbio.2008.10.006
Piacente S, Masullo M, De Neve N, Dewelle J, Hamed A, Kiss R, Mijatovic T (2009) Cardenolides from Pergularia tomentosa display cytotoxic activity resulting from their potent inhibition of Na/K-ATPase. J Nat Prod 72(6):1087–1091. https://doi.org/10.1021/np800810f
Todeschini R, Consonni V, Mauri A, Pavan M (2004) Detecting, “bad” regression models: multicriteria fitness functions in regression analysis. Anal Chem Acta 515:199–208. https://doi.org/10.1016/j.aca.2003.12.010
Veerasamy R, Rajak H, Jain A, Sivadasan S, Varghese CP, Agrawal RK (2011) Validation of QSAR models—strategies and importance. Int J Drug Desing Discov 2(3):511–519
Kier LB, Hall LH (1990) An electrotopological-state index for atoms in molecules. Pharm Res 7(8):801–807. https://doi.org/10.1023/A:1015952613760
Garg P, Verma J, Roy N (2008) In silico modeling for blood—brain barrier permeability predictions. In: Ehrhardt C, Kim KJ (eds) Drug absorption studies. In situ, in vitro and in silico models. Biotechnology pharmaceutical aspects. Springer, New York, pp 510–556. https://doi.org/10.1007/978-0-387-74901-3. ISBN 978-0-387-74900-6
Yatime L, Laursen M, Morth JP, Esmann M, Nissen P, Fedosova NU (2011) Structural insights into the high-affinity binding of cardiotonic steroids to the Na + , K + -ATPase. J Struct Biol 174(2):296–306. https://doi.org/10.1016/j.jsb.2010.12.004
Zhang XH, Zhu HL, Yu Q, Xuang LJ (2007) Cytotoxic cardenolides from Streptocaulon griffithii. Chem Biodivers 4:998–1002. https://doi.org/10.1002/cbdv.200790091
Cao YL, Zhang MH, Lu YF, Li CY, Tang JS, Jiang MM (2018) Cardenolides from the leaves of Nerium oleander. Fitoterapia 127:293–300. https://doi.org/10.1016/j.fitote.2018.03.004
Khatri HR, Bhattarai B, Kaplan W, Li Z, Curtis-Long MJ, Aye Y, Nagorny P (2019) Modular total synthesis and cell-based anticancer activity evaluation of ouabagenin and other cardiotonic steroids with varying degrees of oxygenation. J Am Chem Soc 14(12):4849–4860. https://doi.org/10.1021/jacs.8b12870
Melero CP, Medarde M, San Feliciano A (2000) A short review on cardiotonic steroids and their aminoguanidine analogs. Molecules 5(1):51–81. https://doi.org/10.3390/50100051
Silva IT, Munkert J, Nolte E, Zanchett-Schneider NF, Carvalho-Rocha S, Pacheco-Ramos AC, Kreis W, Castro-Braga F, Maia de Padua R, Taranto AG, Cortes V, Barbosa LA, Wach S, Taubert H, Oliviera-Simoes CM (2018) Cytotoxicity of AMANTADIG: a semisynthetic digitoxigenin derivate—alone and in combination with docetaxel un human hormone-refractory prostate cancer cells and its effect on Na+/K+-ATPase inhibition. Biomed Pharmacother 107:464–474. https://doi.org/10.1016/j.biopha.2018.08.028
Pessôa MTC, Barbosa LA, Villar JAFP (2018) Chapter 3. Synthesis of cardiac steroids and their role in heart failure and cancer. In: Atta-ur- Rahman (ed) Studies in natural products chemistry, vol 57. Elsevier, Amsterdam, pp 79–113. https://doi.org/10.1016/b978-0-444-64057-4.00003-x. ISBN 978-0-444-64057-4
Tian DM, Cheng HY, Jiang MM, Shen WZ, Tang JS, Yao XS (2015) Cardiac glycosides from the seeds of Thevetia peruviana. J Nat Prod 79(38–50):55. https://doi.org/10.1021/acs.jnatprod.5b00611
Laursen M, Gregersen JL, Yatime L, Nissen P, Fedesova NU (2015) Structures and characterization of digoxin and bufalin-bound Na/K-ATPase compared with the ouabain-bound complex. PNAS 112(6):1755–1760. https://doi.org/10.1073/pnas.1422997112
Banuls LM, Urban E, Gelbcke M, Dufrasne F, Kopp B, Kiss R, Zhel M (2013) Structure-Activity Relationship analysis of bufadienolide-induced in vitro growth inhibitory effects on mouse and human cancer cells. J Nat Prod 76:1078–1084. https://doi.org/10.1021/np400034d
Morsy N (2017) Chapter 2. Cardiac glycosides in medicinal plants. In: El-Shamy H (ed) Aromatic and medicinal plants. Back to nature, vol 2, Intech open, Rijeka. https://doi.org/10.5772/65963. ISBN 978-953-51-7348-9
Weiland J, Ritzau M, Megges R, Schön R, Watson TR, Repke KRH (1995) Synthesis of acetates of gomphogenin and gomphoside and evaluation of structure–activity relationships. Eur J Med Chem 30(10):763–767. https://doi.org/10.1016/0223-5234(96)88295-X
Liu R, Sun H, So SS (2001) Development of quantitative structure–property relationship models for early ADME evaluation in drug discovery. 2. Blood–brain barrier penetration. J Chem Inf Comput Sci 41:1623–1632. https://doi.org/10.1021/ci010290i
El-Seedi HR, Khalifa SAM, Taher EA, Farag MA, Saeed A, Gamal M, Hegazy MEF, Youssef D, Musharraf SG, Alajlani MM, Xiao J, Efferth T (2019) Cardenolides: insights from the chemical structure and pharmacological utility. Pharmacol Res 141:123–175. https://doi.org/10.1016/j.phrs.2018.12.015
Cornelius F, Kanai R, Toyoshima C (2013) A structural view on the functional importance of the sugar moiety and steroid hydroxyls of cardiotonic steroids in binding to Na, K-ATPase. J Biol Chem 288(9):6602–6616. https://doi.org/10.1074/jbc.M112.442137
Ogawa H, Shinoda T, Cornelius F, Toyoshima C (2009) Crystal structure of the sodium-potassium pump (Na + , K + -ATPase) with bound potassium and ouabain. Proc Natl Acad Sci USA 106(33):13742–13747. https://doi.org/10.1073/pnas.0907054106
Acknowledgements
This work was partially supported by the Consejo Nacional de Ciencia y Tecnologia (CONACYT, Grant 83462) and the scholarship grant for Meneses-Sagrero (Grant 492204).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflicts of interest
The authors have no conflict of interest to declare.
Ethical statement
This work is a computational study and does not require any ethical statement.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Meneses-Sagrero, S.E., Rascón-Valenzuela, L.A., Sotelo-Mundo, R. et al. Antiproliferative activity of cardenolides on cell line A549: structure–activity relationship analysis. Mol Divers 25, 2289–2305 (2021). https://doi.org/10.1007/s11030-020-10119-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11030-020-10119-w