Abstract
The value of double potential pulse differential techniques for electrochemical studies of dynamic chemical speciation is investigated within the context of liquid|liquid electrochemistry. Then, not only information about the speciation thermodynamics and kinetics is accessible, but also about the species lipophilicity. This provides a comprehensive view of their behaviour in natural and biological media. With the above aim, the current-potential response of the ACDT mechanism (aqueous complexation-dissociation coupled to transfer), where two chemically linked species can transfer between a hydrophilic and a lipophilic phase, is modelled in differential double pulse voltammetry (DDPV) and additive differential pulse voltammetry (ADPV). Explicit closed-form expressions are deduced for both techniques at liquid|liquid macrointerfaces, applicable to any charge number and lipophilicity of the species. The DDPV and ADPV signals are shown to be well sensitive to the features of the chemical reaction, as well as to the species charge and lipophilic character. Also, these techniques offer higher resolution and discrimination against superimposed signals than direct-current techniques, together with high sensitivity and minimization of capacitive effects and background currents, which are key advantages for accurate quantitative analysis.








Similar content being viewed by others
Notes
Note that the TOC scheme is a particular case of the ACDT mechanism.
References
Zhao C-M, Campbell PGC, Wilkinson KJ (2016) When are metal complexes bioavailable? Environ Chem 13(3):425–433
Kot A, Namiesńik J (2000) The role of speciation in analytical chemistry. TrAC Trends Anal Chem 19(2-3):69–79
Kiss T, Enyedy ÉA, Jakusch T (2017) Development of the application of speciation in chemistry. Coord Chem Rev 352:401–423
Lobinski R (1997) Elemental speciation and coupled techniques. Appl Spectrosc 51(7):260A–278A
Sadee B, Foulkes ME, Hill SJ (2015) Coupled techniques for arsenic speciation in food and drinking water: a review. J Anal At Spectrom 30(1):102–118
Zhang H, Davison W (2015) Use of diffusive gradients in thin-films for studies of chemical speciation and bioavailability. Environ Chem 12(2):85–101
Michalke B, Willkommen D, Drobyshev E, Solovyev N (2018) The importance of speciation analysis in neurodegeneration research. TrAC Trends Anal Chem 104:160–170
Oliveira CS, Piccoli BC, Aschner M, Rocha JBT (2017) Chemical speciation of selenium and mercury as determinant of their neurotoxicity. In: Aschner M, Costa LG (eds) Neurotoxicity of metals. Springer, Cham, pp 53–83
Sabri YM, Ippolito SJ, Tardio J, Abd Hamid SB, Bhargava SK (2015) Mercury migration and speciation study during monoethylene glycol regeneration processes. Ind Eng Chem Res 54(19):5349–5355
Córdoba P (2017) Partitioning and speciation of selenium in wet limestone flue gas desulphurisation systems: a review. Fuel 202:184–195
Bard AJ, Faulkner LR (2001) Electrochemical methods : fundamentals and applications. Wiley, New York
Shao Y, Osborne MD, Girault HH (1991) Assisted ion transfer at micro-ITIES supported at the tip of micropipettes. J Electroanal Chem 318(1-2):101–109
Laborda E, Olmos JM, Molina A (2016) Transfer of complexed and dissociated ionic species at soft interfaces: a voltammetric study of chemical kinetic and diffusional effects. Phys Chem Chem Phys 18(15):10158–10172
Molina A, Olmos JM, Laborda E, Gómez-Gil JM, González J (2016) Voltammetry of the aqueous complexation--dissociation coupled to transfer (ACDT) mechanism with charged ligands. Phys Chem Chem Phys 18(25):17091–17104
Hanzlík J, Hovorka J, Camus AM (1987) Transfer of trisbipyridine transition metal complexes across the water-dichloroethane interface. Collect Czechoslov Chem Commun 52(4):838–847
Deryabina MA, Hansen SH, Østergaard J, Jensen H (2009) Effect of α-cyclodextrin on drug distribution studied by electrochemistry at interfaces between immiscible electrolyte solutions. J Phys Chem B 113(20):7263–7269
Baruzzi AM, Wendt H (1990) Interfacial phase transfer of amino-complexed Ni (II) cations: a model for solvent extraction of transition metals. J Electroanal Chem Interfacial Electrochem 279(1-2):19–30
Das NR, Bhattacharyya SN (1976) Solvent extraction of gold. Talanta 23(7):535–540
Ding Z, Reymond F, Baumgartner P, Fermı́n DJ, Brevet PF, Carrupt PA, Girault HH (1998) Mechanism and dynamics of methyl and ethyl orange transfer across the water/1, 2-dichloroethane interface. Electrochim Acta 44(1):3–13
Horrocks BR, Mirkin MV (1998) Cation binding to DNA studied by ion-transfer voltammetry at micropipets. Anal Chem 70(22):4653–4660
Moretto LM, Kalcher K (2014) Environmental analysis by electrochemical sensors and biosensors. Springer, New York
Torralba E, Ortuño JA, Molina A, Serna C, Karimian F (2014) Facilitated ion transfer of protonated primary organic amines studied by square wave voltammetry and chronoamperometry. Anal Chim Acta 826:12–20
Ortuño JA, Olmos JM, Torralba E, Molina A (2016) Sensing and characterization of neurotransmitter 2-phenylethylamine based on facilitated ion transfer at solvent polymeric membranes using different electrochemical techniques. Sensors Actuators B Chem 222:930–936
Ribeiro JA, Miranda IM, Silva F, Pereira CM (2010) Electrochemical study of dopamine and noradrenaline at the water/1, 6-dichlorohexane interface. Phys Chem Chem Phys 12(46):15190–15194
Nishi N, Murakami H, Imakura S, Kakiuchi T (2006) Facilitated transfer of alkali-metal cations by dibenzo-18-crown-6 across the electrochemically polarized interface between an aqueous solution and a hydrophobic room-temperature ionic liquid. Anal Chem 78(16):5805–5812
Reymond F, Lagger G, Carrupt P-A, Girault HH (1998) Facilitated ion transfer reactions across oil|water interfaces: Part II. Use of the convoluted current for the calculation of the association constants and for an amperometric determination of the stoichiometry of MLjz+ complexes. J Electroanal Chem 451(1-2):59–76
Molina A, González J (2016) Pulse voltammetry in physical electrochemistry and electroanalysis. Springer International Publishing, Cham
Molina A, Moreno MM, López-Tenés M, Serna C (2002) Study of an EE mechanism in additive differential pulse techniques. Electrochem Commun 4(5):457–461
Molina A, González J, Laborda E, Compton RG (2012) Additive differential double pulse voltammetry applied to the study of multistep electron transfer reactions with microelectrodes of different geometries. Int J Electrochem Sci 7:5765–5778
Molina A, Moreno MM, Serna C, Camacho L (2001) Additive differential pulse voltammetry, instead of double differential pulse voltammetry. Electrochem Commun 3(7):324–329
Molina A, Morales I (2007) Comparison between derivative and differential pulse voltammetric curves of EC, CE and catalytic processes at spherical electrodes and microelectrodes. Int J Electrochem Sci 2:386–405
Molina A, Morales I, López-Tenés M (2006) Chronoamperometric behaviour of a CE process with fast chemical reactions at spherical electrodes and microelectrodes. Comparison with a catalytic reaction. Electrochem Commun 8:1062–1070
Martínez-Ortiz F, Zoroa N, Molina A, Serna C, Laborda E (2009) Electrochemical digital simulations with an exponentially expanding grid: general expressions for higher order approximations to spatial derivatives: the special case of four-point formulas and their application to multipulse techniques in planar and any. Electrochim Acta 54(3):1042–1055
Girault HH (2011) Electrochemical database-Gibbs energies of transfer. http://sbsrv7.epfl.ch/instituts/isic/lepa/cgi/DB/InterrDB.pl. Accessed 28 Mar 2020
Sakae H, Nagatani H, Imura H (2016) Ion transfer and adsorption behavior of ionizable drugs affected by PAMAM dendrimers at the water|1,2- dichloroethane interface. Electrochim Acta 191:631–639
Morales I, Molina A (2006) Analytical expressions of the I--E--t curves of a CE process with a fast chemical reaction at spherical electrodes and microelectrodes. Electrochem Commun 8(9):1453–1460
Molina A, Ortuño JA, Serna C, Torralba E (2010) Physical insights of salt transfer through solvent polymeric membranes by means of electrochemical methods. Phys Chem Chem Phys 12(40):13296–13303
Acknowledgements
AM and EL greatly appreciate the financial support provided by the Fundacion Séneca de la Región de Murcia (Project 19887/GERM/15). JMO indicates that this work is the result of a grant for the posdoctoral training and development abroad, funded by the Counseling of Employment, Business and Environment of the CARM, through the Fundacion Séneca de la Región de Murcia.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 540 kb)
Appendix
Appendix
Variables
- A :
-
Area of the interface
- c ∗ :
-
Sum of the initial concentrations of transferable species in the aqueous phase
- \( {c}_{\mathrm{i}}^{\ast } \) :
-
Initial equilibrium concentration of species i in the aqueous phase (i = X, XL, L)
- \( {D}_{\mathrm{i}}^{\upalpha} \) :
-
Diffusion coefficient of species i in phase α(= w, o)
- D α :
-
Diffusion coefficient of the free and complexed species in phase α (\( {D}^{\upalpha}={D}_{\mathrm{X}}^{\upalpha}={D}_{\mathrm{X}\mathrm{L}}^{\upalpha} \))
- E 1 :
-
First potential pulse applied in DDPV and ADPV
- E 1/2 :
-
Half-wave potential
- E c :
-
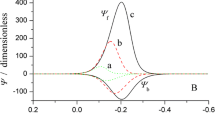
Zero-crossing potential in ADPV
- E peak :
-
Peak potential in DDPV
- E valley :
-
Potential of the valley between two peaks in DDPV
- F :
-
Faraday constant
- I 1 :
-
Current measured at the end of the first potential pulse
- I 2 :
-
Current measured at the end of the second potential pulse
- I ADPV :
-
Current response in ADPV
- \( {I}_{\mathrm{lim}}^{(1)} \) :
-
Limiting current of the first wave obtained in NPV
- I lim :
-
Total limiting current of the NPV curve
- k 1 :
-
Forward rate constant (M−1s‐1)
- k 2 :
-
Backward rate constant (s‐1)
- K :
-
Apparent equilibrium constant under excess of ligand (\( ={K}_{\mathrm{c}}{c}_{\mathrm{L}}^{\ast } \))
- K c :
-
Chemical equilibrium constant based on concentrations (M−1)
- R :
-
Molar gas constant
- T :
-
Absolute temperature
- t :
-
Electrolysis time
- z i :
-
Ionic charge number of species i
- \( {\Delta}_{\mathrm{O}}^{\mathrm{W}}{\phi}_{\mathrm{i}}^{0\prime } \) :
-
Formal transfer potential of species i
- ΔE:
-
Pulse amplitude
- ΔIDDPV:
-
Current response in DDPV
- τ :
-
Duration of the potential pulses applied in NPV
- τ 1 :
-
Duration of the first potential pulse applied in DDPV and ADPV
- τ 2 :
-
Duration of the second potential pulse applied in DDPV and ADPV
Functions
Rights and permissions
About this article
Cite this article
Olmos, J.M., Laborda, E. & Molina, A. Differential double pulse voltammetry (DDPV) and additive differential pulse voltammetry (ADPV) applied to the study of the ACDT mechanism. J Solid State Electrochem 24, 2819–2831 (2020). https://doi.org/10.1007/s10008-020-04619-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-020-04619-w