Abstract
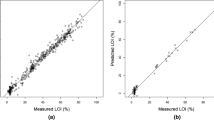
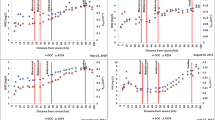
Cyanobacterial blooms are increasing worldwide and have negative impacts on aquatic ecosystems and the services they provide to human societies. A lack of long-term environmental monitoring data, however, has prevented the development of a baseline perspective against which drivers of the increasing frequency and severity of cyanobacterial blooms can be identified. In this study, we evaluate application of spectroscopy-based models to infer historical trends in cyanobacterial abundance from lake sediment cores. Using an amendment series (n = 15) of a sediment matrix spiked with increasing amounts of mixed cyanobacterial culture from 0 to 50 parts per thousand (‰), taxonomically diagnostic carotenoids were measured using visible near-infrared reflectance spectroscopy (VNIRS) and conventional but more costly and time-consuming high-performance liquid chromatography (HPLC). A partial least squares regression model was developed to correlate amendment series VNIR spectra to ‰ of added cyanobacteria. Despite challenges in differentiating carotenoid pigments because of overlapping absorption peaks, applications of the resulting 2-component model (r2 = 0.93, RMSEP = 0.23‰) to sediment cores from four Ontario lakes yielded temporal trends that were significantly correlated with downcore HPLC measures of cyanobacterial pigments in three out of four cases. Although our method is simplistic and may be improved in the future with more complex algorithms employing derivative analysis, we present our results as a possible stepping-stone towards spectral reconstruction of cyanobacterial production. Our study provides proof-of-concept that refinement of a method applying VNIRS to detect cyanobacterial carotenoids in lake sediments has the potential to be an important, rapid and non-destructive assessment tool for research and management of cyanobacterial blooms.





Similar content being viewed by others
References
Butz C, Grosjean M, Poraj-Górska A, Enters D, Tylmann W (2016) Sedimentary bacteriophyophytin a as an indicator of meromixis in varved lake sediments of Lake Jaczno, north-east Poland, CE 1891–2010. Glob Planet Change 144:109–118
Das B, Vinebrooke RD, Sanchez-Azofeifa A, Rivard B, Wolfe AP (2005) Inferring sedimentary chlorophyll concentrations with reflectance spectroscopy: a novel approach to reconstructing historical changes in the trophic status of mountain lakes. Can J Fish Aquat Sci 62:10671078
Favot EJ, Rühland KM, DeSellas AM, Ingram R, Paterson AM, Smol JP (2019) Climate variability promotes unprecedented cyanobacterial blooms in a remote, oligotrophic Ontario lake: evidence from paleolimnology. J Paleolimnol 62:31–52
Fay P (1983) The blue-greens: (cyanophyta-cyanobacteria), vol 160. Institute of Biology’s Studies in Biology, London
Glew JR (1989) A new trigger mechanism for sediment samplers. J Paleolimnol 2:241–243
Graham JL, Loftin KA, Kamman N (2009) Monitoring recreational freshwaters. North American Lake Management Society, LakeLine Summer 8–24
Guillard RRL (1975) Culture of phytoplankton for feeding marine invertebrates. In: Smith WL, Chanley MH (eds) Culture of marine invertebrate animals. Plenum Press, New York, pp 26–60
Halvorson RA, Leng W, Vikesland PJ (2011) Differentiation of microcystin, nodularin, and their component amino acids by drop-coating deposition Raman spectroscopy. Anal Chem 83:9273–9280
Heilbron IM (1942) Some aspects of algal chemistry. Nature 149:398–400
Huisman J, Codd GA, Paerl HW, Ibelings BW, Verspagen JMH, Visser PM (2018) Cyanobacterial blooms. Nat Rev Microbiol 16:471–483
Jeffrey SW, Mantoura RFC, Wright SW (2005) Phytoplankton pigments in oceanography. UNESCO, Paris
Karmakar M, Leavitt PR, Cumming BF (2015) Enhanced algal abundance in northwest Ontario (Canada) lakes during the warmer early-to mid-Holocene period. Quat Sci Rev 123:168–179
Koreivienė J, Anne O, Kasperovičienė J, Burškytė V (2014) Cyanotoxin management and human health risk mitigation in recreational waters. Environ Monit Assess 186:4443–4459
Korsman T, Dåbakk E, Nilsson MB (2001) Near-infrared spectrometry (NIRS) in palaeolimnology. In: Last WM, Smol JP (eds) Tracking environmental change using lake sediments. Volume 2: Physical and geochemical methods. Kluwer Academic Publishers, Dordrecht
Kotai J (1972) Instructions for preparation of modified nutrient solution Z8 for algae. Norwegian Institute for Water Research, Blindern, Oslo 11/69
Leavitt PR, Hodgson DA (2001) Sedimentary pigments. In: Smol JP, Birks HJB, Last WM (eds) Tracking environmental change using lake sediments. Volume 3: Terrestrial, algal, and siliceous indicators. Kluwer Academic Publishers, Dordrecht
Malley DF, Williams PC (1997) Use of near-infrared reflectance spectroscopy in prediction of heavy metals in freshwater sediment by their association with organic matter. Environ Sci Technol 31:3461–3467
Malley DF, Lockhart L, Wilkinson P, Hauser B (2000) Determination of carbon, carbonate, nitrogen, and phosphorus in freshwater sediments by near-infrared reflectance spectroscopy; rapid analysis and a check on conventional analytical methods. J Paleolimnol 24:415–425
McGowan S, Britton G (1999) Ancient blue-green blooms. Limnol Oceanogr 44:436–439
Mevik BH, Wehrens R, Liland KH, Hiemstra P (2013) pls: partial least squares and principal component regression. R package version (2.4-3). https://CRAN.R-project.org/package=pls
Meyer-Jacob C, Michelutti N, Paterson AM, Monteith D, Yang H, Weckström J, Smol JP, Bindler R (2017) Inferring past trends in lake water organic carbon concentrations in northern lakes using sediment spectroscopy. Environ Sci Technol 51:13248–13255
Meyer-Jacob C, Michelutti N, Paterson AM, Cumming BF, Smol JP (2019) The browning and re-browning of lakes: divergent lake-water organic carbon trends linked to acid deposition and climate change. Sci Rep 9:16676
Michelutti N, Smol JP (2016) Visible spectroscopy reliably tracks trends in paleo-production. J Paleolimnol 56:253–265
Michelutti N, Blais JM, Cumming BF, Paterson AM, Rühland K, Wolfe AP, Smol JP (2010) Do spectrally inferred determinations of chlorophyll a reflect trends in lake trophic status? J Paleolimnol 43:205–217
Moir KE, Hickey MBC, Leavitt PR, Ridal JJ, Cumming BF (2018) Paleolimnological proxies reveal continued eutrophication issues in the St. Lawrence River Area of Concern. J Gt Lakes Res 44:357–366
Morton RA (1975) Biochemical spectroscopy. Wiley, New York
Nicholls S, Crompton J (2018) A comprehensive review of the evidence of the impact of surface water quality on property values. Sustainability 10:500
O’Neil JM, Davis TW, Burford MA, Gobler CJ (2012) The rise of harmful cyanobacterial blooms: the potential roles of eutrophication and climate change. Harmful Algae 14:313–334
Paterson AM, Winter JG, Nicholls KH, Clark BJ, Ramcharan CW, Yan ND, Somers KM (2008) Long-term changes in phytoplankton composition in seven Canadian Shield lakes in response to multiple anthropogenic stressors. Can J Fish Aquat Sci 65:846–861
Pick FR (2016) Blooming algae: a Canadian perspective on the rise of toxic cyanobacteria. Can J Fish Aquat Sci 73:1149–1158
R Core Team (2018) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. http://www.R-project.org/
Rinnan A, van den Berg F, Engelsen SB (2009) Review of the most common pre-processing techniques for near-infrared spectra. Trends Anal Chem 28:1201–1222
Rosén P (2005) Total organic carbon (TOC) of lake water during the Holocene inferred from lake sediments and near-infrared spectroscopy (NIRS) in eight lakes from northern Sweden. Biogeochemistry 76:503–516
Rosén P, Dåbakk E, Renberg I, Nilsson M, Hall R (2000) Near-infrared spectrometry (NIRS): a new tool for inferring past climatic changes from lake sediments. Holocene 10:161–166
Rouillard A, Rosén P, Douglas MSV, Pienitz R, Smol JP (2011) A model for inferring dissolved organic carbon (DOC) in lakewater from visible-near-infrared spectroscopy (VNIRS) measures in lake sediment. J Paleolimnol 46:187–202
Schindler DW, Hecky RE, Findlay DL, Stainton MP, Parker BR, Paterson MJ, Beaty KG, Lyng M, Kasian SEM (2008) Eutrophication of lakes cannot be controlled by reducing nitrogen input: results of a 37-year whole-ecosystem experiment. Proc Natl Acad Sci USA 32:11254–11258
Smol JP (2008) Pollution of lakes and rivers: a paleoenvironmental perspective, 2nd edn. Wiley, Oxford, p 383
Staub R (1961) Ernährungsphysiologisch-autökologische Untersuchungen an Oscillatoria rubescens D.C. Schweiz Z Hydrol 23:82–198
Takaichi S (2011) Carotenoids in algae: distributions, biosynthesis and functions. Mar Drugs 9:1101–1118
Taranu ZE, Gregory-Eaves I, Leavitt PR, Bunting L, Buchaca T, Catalan J, Domaizon I, Guilizzoni P, Lami A, McGowan S, Moorhouse H, Morabito G, Pick FR, Stevenson MR, Thompson PL, Vinebrooke RD (2015) Acceleration of cyanobacterial dominance in north temperate-subarctic lakes during the Anthropocene. Ecol Lett 18:375–384
Taylor TK, Keel K, Ladsberg JH, Miller M, Backer LC (2013) Canine cyanotoxin poisonings in the United States (1920s–2012): review of suspected and confirmed cases from three data sources. Toxins 5:1597–1628
Thrane JE, Kyle M, Striebel M, Haande S, Grung M, Rohrlack T, Anderson T (2015) Spectrophotometric analysis of pigments: a critical assessment of a high-throughput method for analysis of algal pigment mixtures by spectral deconvolution. PLoS ONE 10:e0137645
Vinebrooke RD, Hall RI, Leavitt PR, Cumming BF (1998) Fossil pigments as indicators of phototrophic response to salinity and climatic change in lakes of western Canada. Can J Fish Aquat Sci 55:668–681
Waters MN, Schelske CL, Kenney WF, Chapman AD (2005) The use of sedimentary algal pigments to infer historic algal communities in Lake Apopka, Florida. J Paleolimnol 33:53–71
Winter JG, DeSellas AM, Fletcher R, Heintsch L, Morley L, Nakamoto L, Utsumi K (2011) Algal blooms in Ontario, Canada: increases in reports since 1994. Lake Reservoir Manag 27:107–114
Wolfe AP, Vinebrooke RD, Michelutti N, Rivard B, Das B (2006) Experimental calibration of lake-sediment spectral reflectance to chlorophyll a concentrations: methodology and paleolimnological validation. J Paleolimnol 36:91–100
Wood SN (2011) Fast stable restricted maximum likelihood and marginal likelihood estimation of semiparametric generalized linear models. J R Stat Soc B 73:3–36
Wood SN (2017) Generalized additive models: an introduction with R, 2nd edn. Chapman and Hall/CRC, Boca Raton
Acknowledgements
We gratefully acknowledge Carsten Meyer-Jacob for helpful insights regarding early versions of this manuscript and on spectral detection of sediment properties, in general. We also thank Moumita Karmakar, Katherine Moir, Brian Cumming, and Peter Leavitt for providing downcore HPLC pigment data. Four anonymous reviewers provided critiques that greatly improved this manuscript. Financial support was provided by an NSERC CREATE ABATE grant awarded to J.P.S., an NSERC Discovery Grant to A.M.P., and an NSERC Industrial Research and Development (Postdoctoral) Fellowship to K.R.H.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Favot, E.J., Hadley, K.R., Paterson, A.M. et al. Using visible near-infrared reflectance spectroscopy (VNIRS) of lake sediments to estimate historical changes in cyanobacterial production: potential and challenges. J Paleolimnol 64, 335–345 (2020). https://doi.org/10.1007/s10933-020-00140-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10933-020-00140-2