Abstract
Introduction
While monophasic and relapsing forms of myelin oligodendrocyte glycoprotein antibody associated disorders (MOGAD) are increasingly diagnosed world-wide, consensus on management is yet to be developed.
Objective
To survey the current global clinical practice of clinicians treating MOGAD.
Method
Neurologists worldwide with expertise in treating MOGAD participated in an online survey (February–April 2019).
Results
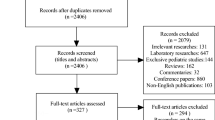
Fifty-two responses were received (response rate 60.5%) from 86 invited experts, comprising adult (78.8%, 41/52) and paediatric (21.2%, 11/52) neurologists in 22 countries. All treat acute attacks with high dose corticosteroids. If recovery is incomplete, 71.2% (37/52) proceed next to plasma exchange (PE). 45.5% (5/11) of paediatric neurologists use IV immunoglobulin (IVIg) in preference to PE. Following an acute attack, 55.8% (29/52) of respondents typically continue corticosteroids for ≥ 3 months; though less commonly when treating children. After an index event, 60% (31/51) usually start steroid-sparing maintenance therapy (MT); after ≥ 2 attacks 92.3% (48/52) would start MT. Repeat MOG antibody status is used by 52.9% (27/51) to help decide on MT initiation. Commonly used first line MTs in adults are azathioprine (30.8%, 16/52), mycophenolate mofetil (25.0%, 13/52) and rituximab (17.3%, 9/52). In children, IVIg is the preferred first line MT (54.5%; 6/11). Treatment response is monitored by MRI (53.8%; 28/52), optical coherence tomography (23.1%; 12/52) and MOG antibody titres (36.5%; 19/52). Regardless of monitoring results, 25.0% (13/52) would not stop MT.
Conclusion
Current treatment of MOGAD is highly variable, indicating a need for consensus-based treatment guidelines, while awaiting definitive clinical trials.








Similar content being viewed by others
Availability of data and material
The survey can be viewed in its original format in the online supplementary material. Complete results are available upon reasonable request to the corresponding author.
References
O’Connor KC, McLaughlin KA, De Jager PL et al (2007) Self-antigen tetramers discriminate between myelin autoantibodies to native or denatured protein. Nat Med 13(2):211–217. https://doi.org/10.1038/nm1488
Waters P, Woodhall M, O’Connor KC et al (2015) MOG cell-based assay detects non-MS patients with inflammatory neurologic disease. Neurol Neuroimmunol Neuroinflamm 2(3):e89. https://doi.org/10.1212/NXI.0000000000000089
Kitley J, Woodhall M, Waters P et al (2012) Myelin-oligodendrocyte glycoprotein antibodies in adults with a neuromyelitis optica phenotype. Neurology 79(12):1273–1277. https://doi.org/10.1212/WNL.0b013e31826aac4e
Jarius S, Ruprecht K, Kleiter I et al (2016) MOG-IgG in NMO and related disorders: a multicenter study of 50 patients. Part 2: Epidemiology, clinical presentation, radiological and laboratory features, treatment responses, and long-term outcome. J Neuroinflamm 13(1):280. https://doi.org/10.1186/s12974-016-0718-0
Kitley J, Waters P, Woodhall M et al (2014) Neuromyelitis optica spectrum disorders with aquaporin-4 and myelin-oligodendrocyte glycoprotein antibodies: a comparative study. JAMA Neurol 71(3):276–283. https://doi.org/10.1001/jamaneurol.2013.5857
Sato DK, Callegaro D, Lana-Peixoto MA et al (2014) Distinction between MOG antibody-positive and AQP4 antibody-positive NMO spectrum disorders. Neurology 82(6):474–481. https://doi.org/10.1212/WNL.0000000000000101
Pandit L, Sato DK, Mustafa S et al (2016) Relapsing optic neuritis and isolated transverse myelitis are the predominant clinical phenotypes for patients with antibodies to myelin oligodendrocyte glycoprotein in India. Mult Scler J Exp Transl Clin 2:2055217316675634. https://doi.org/10.1177/2055217316675634
Ramanathan S, Mohammad S, Tantsis E et al (2018) Clinical course, therapeutic responses and outcomes in relapsing MOG antibody-associated demyelination. J Neurol Neurosurg Psychiatry 89(2):127–137. https://doi.org/10.1136/jnnp-2017-316880
Ogawa R, Nakashima I, Takahashi T et al (2017) MOG antibody-positive, benign, unilateral, cerebral cortical encephalitis with epilepsy. Neurol Neuroimmunol Neuroinflamm 4:e322. https://doi.org/10.1212/NXI.0000000000000322
Hamid SHM, Whittam D, Saviour M et al (2018) Seizures and encephalitis in myelin oligodendrocyte glycoprotein IgG disease vs aquaporin 4 IgG disease. JAMA Neurol 75(1):65–71. https://doi.org/10.1001/jamaneurol.2017.3196
Fujimori J, Takai Y, Nakashima I et al (2017) Bilateral frontal cortex encephalitis and paraparesis in a patient with anti-MOG antibodies. J Neurol Neurosurg Psychiatry 88(6):534–536. https://doi.org/10.1136/jnnp-2016-315094
Cobo-Calvo A, Ruiz A, D’Indy H et al (2017) MOG antibody-related disorders: common features and uncommon presentations. J Neurol 264(7):1945–1955. https://doi.org/10.1007/s00415-017-8583-z
Hacohen Y, Wong YY, Lechner C et al (2018) Disease course and treatment responses in children with relapsing myelin oligodendrocyte glycoprotein antibody-associated disease. JAMA Neurol 75(4):478–487. https://doi.org/10.1001/jamaneurol.2017.4601
Höftberger R, Sepúlveda M, Armangue T et al (2015) Antibodies to MOG and AQP4 in adults with neuromyelitis optica and suspected limited forms of the disease. Mult Scler 21(7):866–874. https://doi.org/10.1177/1352458514555785
Mariotto S, Ferrari S, Monaco S et al (2017) Clinical spectrum and IgG subclass analysis of anti-myelin oligodendrocyte glycoprotein antibody-associated syndromes: a multicenter study. J Neurol 264(12):2420–2430. https://doi.org/10.1007/s00415-017-8635-4
Jurynczyk M, Messina S, Woodhall MR et al (2017) Clinical presentation and prognosis in MOG-antibody disease: a UK study. Brain 140(2):3128–3138. https://doi.org/10.1093/brain/awx276
Cobo-Calvo A, Ruiz A, Maillart E et al (2018) Clinical spectrum and prognostic value of CNS MOG autoimmunity in adults: the MOGADOR study. Neurology 90(21):e1858–e1869. https://doi.org/10.1212/WNL.0000000000005560
Juryńczyk M, Jacob A, Fujihara K, Palace J (2019) Myelin oligodendrocyte glycoprotein (MOG) antibody-associated disease: practical considerations. Pract Neurol 19(3):187–195. https://doi.org/10.1136/practneurol-2017-001787
Hacohen Y, Banwell B (2019) Treatment approaches for MOG-Ab-associated demyelination in children. Curr Treat Options Neurol 21(1):2. https://doi.org/10.1007/s11940-019-0541-x
Wynford-Thomas R, Jacob A, Tomassini V (2019) Neurological update: MOG antibody disease. J Neurol 266(5):1280–1286. https://doi.org/10.1007/s00415-018-9122-2
Jarius S, Paul F, Asgari N et al (2018) MOG encephalomyelitis: international recommendations on diagnosis and antibody testing. J Neuroinflamm 15(1):134. https://doi.org/10.1186/s12974-018-1144-2
Dos Passos GR, Oliveira LM, da Costa BK et al (2018) MOG-IgG-Associated Optic neuritis, encephalitis, and myelitis: lessons learned from neuromyelitis optica spectrum disorder. Front Neurol 9:217. https://doi.org/10.3389/fneur.2018.00217
Borisow N, Mori M, Kuwabara S, Scheel M, Paul F (2018) Diagnosis and treatment of NMO spectrum disorder and MOG-encephalomyelitis. Front Neurol 9:888. https://doi.org/10.3389/fneur.2018.00888
Pandit L, Mustafa S, Nakashima I, Takahashi T, Kaneko K (2018) MOG-IgG-associated disease has a stereotypical clinical course, asymptomatic visual impairment and good treatment response. Mult Scler J Exp Transl Clin 4(3):2055217318787829. https://doi.org/10.1177/2055217318787829
Montcuquet A, Collongues N, Papeix C et al (2017) Effectiveness of mycophenolate mofetil as first-line therapy in AQP4-IgG, MOG-IgG, and seronegative neuromyelitis optica spectrum disorders. Mult Scler 23(10):1377–1384. https://doi.org/10.1177/1352458516678474
Sellner J, Boggild M, Clanet M et al (2010) EFNS guidelines on diagnosis and management of neuromyelitis optica. Eur J Neurol 17(8):1019–1032. https://doi.org/10.1111/j.1468-1331.2010.03066.x
Palace J, Leite MI, Jacob A (2012) A practical guide to the treatment of neuromyelitis optica. Pract Neurol 12(4):209–214. https://doi.org/10.1136/practneurol-2012-000237
Kimbrough DJ, Fujihara K, Jacob A et al (2012) Treatment of neuromyelitis optica: review and recommendations. Mult Scler Relat Disord 1(4):180–187. https://doi.org/10.1016/j.msard.2012.06.002
Jarius S, Ruprecht K, Kleiter I et al (2016) MOG-IgG in NMO and related disorders: a multicenter study of 50 patients. Part 1: Frequency, syndrome specificity, influence of disease activity, long-term course, association with AQP4-IgG, and origin. J Neuroinflamm 13(1):279. https://doi.org/10.1186/s12974-016-0717-1
Hyun JW, Woodhall MR, Kim SH et al (2017) Longitudinal analysis of myelin oligodendrocyte glycoprotein antibodies in CNS inflammatory diseases. J Neurol Neurosurg Psychiatry 88(10):811–817. https://doi.org/10.1136/jnnp-2017-315998
Spadaro M, Gerdes LA, Krumbholz M et al (2016) Autoantibodies to MOG in a distinct subgroup of adult multiple sclerosis. Neurol Neuroimmunol Neuroinflamm 3(5):e257. https://doi.org/10.1212/NXI.0000000000000257
Cobo-Calvo A, Sepúlveda M, D’Indy H et al (2019) Usefulness of MOG-antibody titres at first episode to predict the future clinical course in adults. J Neurol 266(4):800–815. https://doi.org/10.1007/s00415-018-9160-9
Hennes E, Baumann M, Schanda K et al (2017) Prognostic relevance of MOG antibodies in children with an acquired demyelinating syndrome. Neurology 89(9):900–908. https://doi.org/10.1212/WNL.0000000000004312
Waters P, Fadda G, Woodhall M et al (2019) Serial anti-myelin oligodendrocyte glycoprotein antibody analysis and outcomes in children with demyelinating syndromes. JAMA Neurol. https://doi.org/10.1001/jamaneurol.2019.2940 (Epub ahead of print)
Duignan S, Wright S, Rossor T et al (2018) Myelin oligodendrocyte glycoprotein and aquaporin-4 antibodies are highly specific in children with acquired demyelinating syndromes. Dev Med Child Neurol 60:958–962. https://doi.org/10.1111/dmcn.13703
Cobo-Calvo A, Sepúlveda M, Rollot F et al (2019) Evaluation of treatment response in adults with relapsing MOG-Ab-associated disease. J Neuroinflamm 16(1):134. https://doi.org/10.1186/s12974-019-1525-1
Wong YYM, Hacohen Y, Armangue T et al (2018) Paediatric acute disseminated encephalomyelitis followed by optic neuritis: disease course, treatment response and outcome. Eur J Neurol 25(5):782–786. https://doi.org/10.1111/ene.13602
Whittam DH, Cobo-Calvo A, Lopez-Chiriboga AS et al (2018) Treatment of MOG-IgG-associated demyelination with Rituximab: a multinational study of 98 patients (S13.003). Talk presented at: 70th American Academy of Neurology Annual Meeting, Los Angeles, USA, 21–27 April, 2018
Okuda Y, Sakoda S, Fujimura H, Saeki Y, Kishimoto T, Yanagihara T (1999) IL-6 plays a crucial role in the induction phase of myelin oligodendrocyte glycoprotein 35–55 induced experimental autoimmune encephalomyelitis. J Neuroimmunol 101(2):188–196. https://doi.org/10.1016/s0165-5728(99)00139-3
Kaneko K, Sato DK, Nakashima I et al (2018) CSF cytokine profile in MOG-IgG+ neurological disease is similar to AQP4-IgG+ NMOSD but distinct from MS: a cross-sectional study and potential implications. J Neurol Neurosurg Psychiatry 89(9):927–936. https://doi.org/10.1136/jnnp-2018-317969
Kothur K, Wienholt L, Tantsis EM et al (2016) B cell, Th17, and neutrophil related cerebrospinal fluid cytokine/chemokines are elevated in MOG antibody associated demyelination. PLoS ONE 11(2):e0149411. https://doi.org/10.1371/journal.pone.0149411
Novi G, Gastaldi M, Franciotta D, Pesce G, Benedetti L, Uccelli A (2019) Tocilizumab in MOG-antibody spectrum disorder: a case report. Mult Scler Relat Disord 27:312–314. https://doi.org/10.1016/j.msard.2018.11.012
Hayward-Könnecke H, Reindl M, Martin R, Schippling S (2019) Tocilizumab in severe recurrent anti-MOG-associated optic neuritis. Neurology 92(16):765–767. https://doi.org/10.1212/WNL.0000000000007312
Ringelstein M, Kleiter I, Rommer P et al (2019) Long-term interleukin-6-receptor blockade in neuromyelitis optica spectrum disorder and MOG associated encephalomyelitis (P1344). Poster presented at: 35th Congress of the European Committee for Treatment and Research in Multiple Sclerosis, Stockholm, Sweden, 11–13 September, 2019
Mader S, Gredler V, Schanda K et al (2011) Complement activating antibodies to myelin oligodendrocyte glycoprotein in neuromyelitis optica and related disorders. J Neuroinflamm 8:184
Peschl P, Schanda K, Zeka B et al (2017) Human antibodies against the myelin oligodendrocyte glycoprotein can cause complement-dependent demyelination. J Neuroinflamm 14(1):208. https://doi.org/10.1186/s12974-017-0984-5
Fang L, Kang X, Wang Z et al (2019) Myelin oligodendrocyte glycoprotein-IgG contributes to oligodendrocytopathy in the presence of complement, distinct from astrocytopathy induced by AQP4-IgG. Neurosci Bull 35(5):853–866. https://doi.org/10.1007/s12264-019-00375-8
Chamberlain JL, Huda S, Whittam DH, Matiello M, Morgan BP, Jacob A (2019) Role of complement and potential of complement inhibitors in myasthenia gravis and neuromyelitis optica spectrum disorders: a brief review. J Neurol. https://doi.org/10.1007/s00415-019-09498-4 (Epub ahead of print)
Wildemann B, Jarius S, Schwarz A et al (2017) Failure of alemtuzumab therapy to control MOG encephalomyelitis. Neurology 89(2):207–209. https://doi.org/10.1212/WNL.0000000000004087
Funding
The UK Neuromyelitis Optica Diagnostic and Advisory Service is funded by the Highly Specialised Commissioning Division of NHS England. There was no formal sponsorship for this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
D.H. Whittam, E.Gibbons, V. Karthikeayan, R. Kneen, S. Chandratre, J. de Seze, K. Deiva, R.Q. Hintzen, I. Kleiter, K. Rostasy, P. Huppke, F. Paul, A.K. Pröbstel, M.P. Amato, M. Nosadini, M.M. Mancardi, Z. Illes, A. Siva, G. Akman-Demir, L. Pandit, M. Apiwattankul, J.Y. Hor, S. Viswanathan, W. Qiu, H.J. Kim, I. Nakashima, R.C. Dale, M. Boggild, S. Broadley, M.A. Lana-Peixoto, P. Cabre, B.G. Weinshenker, B. Greenberg, M. Matiello, E.C. Klawiter, J.L. Bennett, A.I. Wallach, I. Kister, B.L. Banwell, D. Pohl, M.Levy, M.I. Leite, T. Solomon: nothing to disclose. O. Ciccarelli is a consultant for Roche, Novartis, Teva, Biogen and Merck. B Wildemann has received research grants and/or honoria from Merck Serono, Biogen, Teva, Novartis, Sanofi Genzyme, Bayer Healthcare, and research grants from Bundesministerium für Bildung und Forschung, Deutsche Forschungsgemeinschaft, Dietmar Hopp Foundation and the Klaus Tschira Foundation. S. Jarius’s work was indirectly supported by research grants from Dietmar Hopp Stiftung and from Merck Serono. I.Kleiter has received speaker honoraria and travel funding from Bayer, Biogen, Novartis, Merck, Sanofi Genzyme, Roche; speaker honoraria from Mylan; travel funding from the Guthy-Jackson Charitable Foundation; consulted for Alexion, Bayer, Biogen, Celgene, Chugai, IQVIA, Novartis, Merck, Roche; and research support from Chugai, Diamed. B. Hemmer has served on scientific advisory boards for F. Hoffmann-La Roche Ltd, Novartis, and Bayer AG; he has served as DMSC member for AllergyCare and TG Therapeutics; he or his institution have received speaker honoraria from Medimmune, Novartis, Desitin, and F. Hoffmann-La Roche Ltd; his institution has received research support from Chugai Pharmaceuticals; holds part of two patents; one for the detection of antibodies and T cells against KIR4.1 in a subpopulation of MS patients and one for genetic determinants of neutralizing antibodies to interferon β. O. Aktas reports grants from the German Research Foundation (DFG) and the German Ministry of Education and Research (BMBF), grants and personal fees from Bayer HealthCare, Biogen, Genzyme, Novartis, Teva and Viela Bio, and personal fees from Almirall, MedImmune, Merck Serono and Roche. G. Arrambide has received compensation for consulting services or participation in advisory boards from Sanofi, Merck, and Roche; research support from Novartis; travel expenses for scientific meetings from Novartis, Roche, Stendhal, and ECTRIMS; and speaking honoraria from Sanofi, Merck, and Novartis. M. Tintore has received compensation for consulting services and speaking honoraria from Almirall, Bayer Schering Pharma, Biogen-Idec, Genzyme, Merck-Serono, Novartis, Roche, Sanofi-Aventis, and Teva Pharmaceuticals. MT is co-editor of Multiple Sclerosis Journal-ETC. M. Capobianco received personal honoraria for speaking at meeting or participating in advisory boards from Biogen, Merck, Novartis, Roche, Sanofi, Teva. A. Altintas received travel grants and/or speaker honoraria from Merck, Generica and Novartis. H.J. Kim received research support from the Ministry of Science and ICT, Genzyme, Merck Serono, Teva-Handok, and UCB; received consultancy/speaker fees from Celltrion, Eisai, HanAll BioPharma, MedImmune, Merck Serono, Novartis, Sanofi Genzyme, Teva-Handok, and UCB; serves on a steering committee for MedImmune/VielaBio; is a co-editor for the Multiple Sclerosis Journal—Experimental, Translational, and Clinical, and an associated editor for the Journal of Clinical Neurology. K. Fujihara received consultancy/speaker fees: Alexion Pharmaceuticals, Chugai, Asahi Kasei Medical, Biogen, Eisai, Mitsubishi-Tanabe Pharma, Nihon, Novartis Pharmaceuticals, ONO Pharmaceutical, Takeda, and Teijin. S. Ramanathan has received an Early Career Fellowship from the National Health and Medical Research Council (Australia). D.K. Sato has received a Grants-in-Aid for Scientific Research from the Japan Society for the Promotion of Science (KAKENHI 15K19472); research support from CNPq/Brasil (425331/2016-4), FAPERGS/MS/CNPq/SESRS (17/2551-0001391-3) PPSUS/Brazil, TEVA (research grant for EMOCEMP Investigator Initiated Study), and Euroimmun AG (Neuroimmunological Complications associated with Arboviruses); and speaker honoraria from Biogen, Novartis, Genzyme, TEVA, Merck-Serono, Roche, and Bayer and has participated in advisory boards for Shire, Roche, TEVA, Merck-Serono and Quest/Athena Diagnostics. S. Tenembaum serves as a non-remunerated editorial board member of Neurology: Neuroimmunology & Neuroinflammation. She has received speaker and consulting fees from Biogen-Idec Argentina, Merck Serono LATAM, Genzyme-Sanofi, Novartis, and Teva Neuroscience during the last 3 years. D.M. Wingerchuk received grant support paid to Mayo Clinic from Alexion and TerumoBCT, consultant fees from MedImmune, Celgene, Novartis, and ONO Pharmaceuticals. A. Traboulsee: has received research funding from Chugai, Roche, and Sanofi Genzyme; received honoraria or travel support from Consortium of MS Centers, MS Society of Canada, Biogen, Teva, Roche, Merck/EMD Serono, Sanofi Genzyme, Chugai. J. Palace is partly funded by highly specialised services to run a national congenital myasthenia service and a neuromyelitis optica service. She has received support for scientific meetings and honorariums for advisory work from Merck Serono, Biogen Idec, Novartis, Teva, Chugai Pharma and Bayer Schering, Alexion, Roche, Genzyme, MedImmune, EuroImmun, MedDay, Abide ARGENX, UCB and Viela Bio and grants from Merck Serono, Novartis, Biogen Idec, Teva, Abide, MedImmune, Bayer Schering, Genzyme, Chugai and Alexion. She has received grants from the MS society, Guthrie Jackson Foundation, NIHR, Oxford Health Services Research Committee, EDEN, MRC, GMSI, John Fell and Myaware for research studies. R. Marignier serves on the scientific advisory board for Novartis and Medimmune; received speaker honoraria and travel funding from Novartis, Biogen, Teva, Sanofi-Aventis/Genzyme, Merck. M. Lim has received consultation fees from CSL Behring; received travel grants from Merck Serono; and was awarded educational grants to organize meetings by Novartis, Biogen Idec, Merck Serono and Bayer. S. Huda has received research support from the Neuromyelitis Optica UK charity. A. Jacob served on the scientific advisory board for Shire Pharmaceuticals; received travel funding and/or speaker honoraria from Biogen Idec, Shire, and Terumo BCT; consulted for Shire Pharmaceuticals; and received research support from Biogen, Alexion Pharmaceuticals, NHS, and University of Liverpool.
Rights and permissions
About this article
Cite this article
Whittam, D.H., Karthikeayan, V., Gibbons, E. et al. Treatment of MOG antibody associated disorders: results of an international survey. J Neurol 267, 3565–3577 (2020). https://doi.org/10.1007/s00415-020-10026-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-020-10026-y