Abstract
The constitutional repeating unit structures of poly(pyromellitic dianhydride-co-4,4′-oxydianiline) or polyamiс acid, butyric acid and complexes between butyric acid and one, two or three units of polyamiс acid were calculated using the density functional theory method (DFT B3LYP) on a 6-31G(d,p) basis with the basis set superposition error (BSSE) correction in the program Gaussian 09. The optimised structures of the template and polyamiс acid allowed to establish intermolecular non-covalent interactions during imprinting and, based on the results, develop a method of polyimide-based molecularly imprinted polymer preparation using butyric acid as template. It was shown that the optimum molar ratio of reagents used for the synthesis of molecularly imprinted polymer is 1:1 (interaction energy ∆E = 43.2 kJ/mol). In the presence of a large number of polyamic acid units in prepolymerisation complexes, the self-association of polyamic acid and the steric hindrance, which reduce the stability of the complexes, occur. The simulation results are in good agreement with experimental data. When analysing the energy values and parameters of bonds of the complexes, it was shown that butyric acid interacts with carboxyl groups from units of polyamiс acid via hydrogen bonding of moderate strength. The IR spectra of a complex between structural unit of polyamic acid and butyric acid were also obtained in order to understand the intermolecular interactions responsible for the molecular recognition. According to the IR spectra, it was shown that the vibrations of the C=O and O–H groups of butyric acid and polyamic acid participating in the interaction are weakened; their vibration frequencies are reduced. The polyimide structure was also constructed in the work. It was shown that two polyimide chains interact with each other due to hydrogen bonds between the hydrogen atoms of the benzene rings and the oxygen of the imide groups.




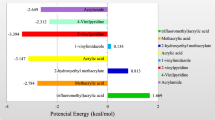
Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Turiel E, Martín-Esteban A (2010) Molecularly imprinted polymers for sample preparation: a review. Anal Chim Acta 668:87–99. https://doi.org/10.1016/j.aca.2010.04.019
Pauling L (1940) A theory of the structure and process of formation of antibodies. J Am Chem Soc 62:2643–2657. https://doi.org/10.1021/ja01867a018
Vlatakis G, Andersson LI, Müller R, Mosbach K (1993) Drug assay using antibody mimics made by molecular imprinting. Nature 361:645–647. https://doi.org/10.1038/361645a0
Speltini A, Scalabrini A, Maraschi F, Sturini M, Profumo A (2017) Newest applications of molecularly imprinted polymers for extraction of contaminants from environmental and food matrices: a review. Anal Chim Acta 974:1–26. https://doi.org/10.1016/j.aca.2017.04.042
Dmitrienko SG, Irkha VV, Kuznetsova AY, Zolotov Yu A (2004) Use of molecular imprinted polymers for the separation and preconcentration of organic compounds. J Anal Chem 59:808–817. https://doi.org/10.1023/B:JANC.0000040694.23348.45
Uzun L, Turner AP (2016) Molecularly-imprinted polymer sensors: realising their potential. Biosens Bioelectron 76:131–144. https://doi.org/10.1016/j.bios.2015.07.013
Pohanka M (2017) Sensors based on molecularly imprinted polymers. Int J Electrochem Sci 12:8082–8094. https://doi.org/10.20964/2017.09.67
Díaz-Díaz G, Diñeiro Y, Menéndez MI, Blanco-López MC, Lobo-Castañón MJ, Miranda-Ordieres AJ, Tuñón-Blanco P (2011) Molecularly imprinted catalytic polymers with biomimetic chloroperoxidase activity. Polymer 52:2468–2473. https://doi.org/10.1016/j.polymer.2011.04.004
Zyablov AN, Kalach AV, Zhibrova Yu A, Selemenev VF, D'yakonova OV (2010) Determination of glycine in aqueous solutions using a molecularly imprinted polymer-modified piezosensor. J Anal Chem 65:91–93. https://doi.org/10.1134/S106193481001017X
Duvanova OV, Zyablov AN, Falaleev AV (2014) Flow-injection determination of oleic and palmitic acid with modified piezoelectric sensors. Sorbtsionnye i khromatograficheskie protsessy 14:691–695 (in Russian)
Zyablov AN, Khalzova SA, Selemenev VF (2017) Sorption of red food coloring polymers with molecular imprints. Izv Vyssh Uchebn Zaved, Khim Khim Tekhnol 60:42–47. (in Russian). https://doi.org/10.6060/tcct.2017607.5595
Lübke C, Lübke M, Whitcombe MJ, Vulfson EN (2000) Imprinted polymers prepared with stoichiometric template – monomer complexes: efficient binding of ampicillin from aqueous solutions. Macromolecules 33:5098–5105. https://doi.org/10.1021/ma000467u
Khan MS, Wate PS, Krupadam RJ (2012) Combinatorial screening of polymer precursors for preparation of benzo [α] pyrene imprinted polymer: an ab initio computational approach. J Mol Model 18:1969–1981. https://doi.org/10.1007/s00894-011-1218-x
Mishina AA, Zyablov AN, Selemenev VF (2010) Modeling of molecularly imprinted polymers for glycine based on polyamic acid and colloxylin. Izv Vyssh Uchebn Zaved, Khim Khim Tekhnol 53:20–24 (in Russian)
Linh CN, Duvanova ОV, Zyablov AN (2019) Application of a molecularly imprinted polymer based on the polyimide as a piezosensor selective coating for determining the oleic acid in oils. Analitika Kontrol 23:120–126 (in Russian). https://doi.org/10.15826/analitika.2019.23.1.006
Mendes TP, Pereira I, Ferreira MR, Chaves AR, Vaz BG (2017) Molecularly imprinted polymer-coated paper as a substrate for highly sensitive analysis using paper spray mass spectrometry: quantification of metabolites in urine. Anal Methods 9:6117–6123. https://doi.org/10.1039/C7AY01648D
D'yakonova OV, Kotov VV, Voishchev VS, Selemenev VF (1998) Ion-exchange properties of polyamide acid films with different degrees of imidization. Russ J Phys Chem A 72:1144–1146
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li H, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery Jr JA, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas O, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2009) GAUSSIAN 09. Gaussian Inc., Wallingford CT
Steiner T (2002) The hydrogen bond in the solid state. Angew Chem Int Ed 41:48–76. https://doi.org/10.1002/1521-3773(20020104)41:1<48::AID-ANIE48>3.0.CO;2-U
Linh CN, Zyablov AN, Duvanova ОV, Selemenev VF (2020) Sorption of carboxylic acids by molecularly imprinted polymers. Izv Vyssh Uchebn Zaved, Khim Khim Tekhnol 63:71–76 (in Russian). https://doi.org/10.6060/ivkkt.20206302.6071
Khan MS, Pal S, Krupadam RJ (2015) Computational strategies for understanding the nature of interaction in dioxin imprinted nanoporous trappers. J Mol Recognit 28:427–437. https://doi.org/10.1002/jmr.2459
Okuyama K, Sakaitani H, Arikawa H (1992) X-ray structure analysis of a thermoplastic polyimide. Macromolecules 25:7261–7267. https://doi.org/10.1021/ma00052a030
Duvanova OV, Sokolova SA, D'yakonova OV, Zyablov AN, Selemenev VF, Kozaderov ОА (2016) Physical and chemical properties and surface morphology of polyimides with palmitic acid molecular imprints. Sorbtsionnye Khromatograficheskie Protsessy 16:610–615 (in Russian)
Funding
This work was supported by the Ministry of Education and Science of the Russian Federation under Grant 02.a03.21.0008.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Code availability
Gaussian 9 software application or custom code.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Linh, C.N., Duvanova, O.V., Yen, V.H. et al. Modeling of butyric acid recognition by molecular imprinted polyimide. J Mol Model 26, 194 (2020). https://doi.org/10.1007/s00894-020-04462-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-020-04462-w