Abstract
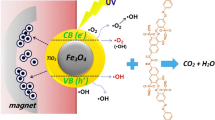
Magnetic graphen oxide (Fe3O4-GO) is applied for preparation of Fe3O4-GO-ZnO (MGOZ) nanocomposite as photocatalyst. The photocatalysts are characterized by FTIR and UV spectrophotometer. FTIR results confirm the presence of Zn–O bonds and Fe–O bonds that are attributed to the ZnO and Fe3O4, respectively. The removal efficiency of methyl orange (MO) is compared using MGOZ and Fe3O4-ZnO at different irradiation time (ranging from 5 to 40 min) and pH (in the range of 3 to 11). The experimental results show that the removal efficiency of MO using MGOZ and Fe3O4-ZnO enhanced with respect to the irradiation time. Meanwhile the lowest and highest removal efficiency are obtained at pH = 7 and pH = 3, respectively. The comparison between removal efficiency of MO using MGOZ and Fe3O4-ZnO reveals that GO has a significant effect on the photocatalytic activity. Meanwhile, the removal efficiency of MO using MGOZ is higher than that of Fe3O4-ZnO. The statistical analysis of results using design of experiments (DOE) and Duncan’s multiple range test at α = 0.05 confirm that irradiation time, pH and their interactions have a significant effect on the removal efficiency of MO.














Similar content being viewed by others
References
L. Gan, H. Li, L. Chen, L. Xu, J. Liu, A. Geng, et al. (2018). Colloid Polym. Sci. 296, (3), 607–615.
Y.-C. Chen, K.-I. Katsumata, Y.-H. Chiu, K. Okada, N. Matsushita, and Y.-J. Hsu (2015). Appl. Catal. A General. 490, 1–9.
Y.-C. Pu, H.-Y. Chou, W.-S. Kuo, K.-H. Wei, and Y.-J. Hsu (2017). Appl. Catal. B Environ. 204, 21–32.
Y.-S. Chang, P.-Y. Hsieh, T.-F. M. Chang, C.-Y. Chen, M. Sone, and Y.-J. Hsu (2020). J. Mater. Chem. A. https://doi.org/10.1039/d0ta02359k.
R. Kumar, R. K. Singh, D. P. Singh, R. Savu, and S. A. Moshkalev (2016). Mater. Design. 111, 291–300.
K.-A. Tsai and Y.-J. Hsu (2015). Appl. Catal. B Environ. 164, 271–278.
K.-A. Tsai, P.-Y. Hsieh, T.-H. Lai, C.-W. Tsao, H. Pan, Y.-G. Lin, et al. (2020). ACS Appl. Energy Mater. 3, 5322–5332.
S. Abbasi (2016). Iran. J. Health Environ. 9, (3), 433–442.
S. Abbasi (2018). Mater. Res. Express. 5, 066302.
S. Abbasi (2020). J. Inorgan. Organometal. Polym. Mater. 30, 1924–1934.
S. Abbasi, F. Ahmadpoor, M. Imani, and M.-S. Ekrami-Kakhki (2020). Int. J. Environ. Anal. Chem. 100, (2), 225–240.
S. Abbasi, M.-S. Ekrami-Kakhki, and M. Tahari (2017). J. Mater. Sci. Mater. Electron. 28, (20), 15306–15312.
S. Abbasi and M. Hasanpour (2017). J. Mater. Sci. Mater. Electron. 28, (2), 1307–1314.
Y.-H. Chiu, T.-F. M. Chang, C.-Y. Chen, M. Sone, and Y.-J. Hsu (2019). Catalysts 9, 430.
N. Roozban, S. Abbasi, and M. Ghazizadeh (2017). J. Mater. Sci. Mater. Electron. 28, (8), 6047–6055. https://doi.org/10.1007/s10854-016-6280-9.
S. Abbasi, M. Hasanpour, and M. S. E. Kakhki (2017). J. Mater. Sci. Mater. Electron. 28, (13), 9900–9910. https://doi.org/10.1007/s10854-017-6745-5.
Y.-H. Chiu, T.-H. Lai, M.-Y. Kuo, P.-Y. Hsieh, and Y.-J. Hsu (2019). Appl. Mater. 7, 080901.
A. Ghaderi, S. Abbasi, and F. Farahbod (2015). Iran. J. Chem. Eng. 12, (3), 96–105.
A. Ghaderi, S. Abbasi, and F. Farahbod (2018). Mater. Res. Express. 5, 065908.
N. Roozban, S. Abbasi, and M. Ghazizadeh (2017). J. Mater. Sci. Mater. Electron. 28, (10), 7343–7352. https://doi.org/10.1007/s10854-017-6421-9.
W. W. Wang, Y. J. Zhu, and L. X. Yang (2007). Adv. Funct. Mater. 17, 59–64.
A. Sanmugam, D. Vikraman, H. J. Park, and A. H.-S. Kim (2017). Nanomaterials 7, 363–376.
M. Azarang, A. Shuhaimi, R. Yousefi, A. M. Golsheikh, and M. Sookhakian (2014). Ceramics Int. 40, 10217–10221.
M. Azarang, A. Shuhaimi, and M. Sookhakian (2015). RSC Adv. 5, 53117–53128.
S. Kurinobu, K. Tsurusaki, Y. Natui, M. Kimata, and M. Hasegawa (2007). J. Magn. Magn. Mater. 310, e1025–e1027.
D. Li, H. Haneda, and J. Photochem (2003). J. Photochem. Photobiol. A. 160, (3), 203–212.
M. Nikazar, M. Alizadeh, R. Lalavi, and M. H. Rostami (2014). Iran. J. Environ. Health Sci. Eng. 12, 21–26.
M. Safari, M. H. Rostami, M. Alizadeh, A. Alizadehbirjandi, S. A. A. Nakhli, and R. Aminzadeh (2014). J. Environ. Health Sci. Eng. 12, (1), 1–10.
D. S. Winatapura, S. H. Dewi, and W. A. Adi (2016). Int. J. Technol. 3, 408–416.
M. Abareshi, E. K. Goharshadi, S. M. Zebarjad, H. K. Fadafan, and A. Youssefi (2010). J. Magn. Magn. Mater. 322, 3895–3901.
A. K. Zak, W. H. A. Majid, M. Darroudi, and R. Yousefi (2011). Mater. Lett. 65, 70–73.
S. Abbasi, S. M. Zebarjad, S. H. N. Baghban, and A. Youssefi (2015). Synth. React. Inorgan. Metal Organ. Nano Metal Chem. 45, 1539–1548.
D. Dastan, N. Chaure, and M. Kartha (2017). J. Mater. Sci. Mater. Electron. 28, 7784–7796.
D. Dastan, S. L. Panahi, and N. B. Chaure (2016). J. Mater. Sci. Mater. Electron. 27, 12291–12296.
A. Akbarzadeh, M. Samiei, S. W. Joo, M. Anzaby, Y. Hanifehpour, H. T. Nasrabadi, et al. (2012). J. Nanobiotechnol. 10, 46–58.
S. Abbasi (2019). Environ. Monitor. Assess. 191, (4), 206–218.
S. Abbasi and M. Hasanpour (2017). J. Mater. Sci. Mater. Electron. 28, (16), 11846–11855. https://doi.org/10.1007/s10854-017-6992-5.
S. Abbasi, M. Hasanpour, F. Ahmadpoor, M. Sillanpää, D. Dastan, and A. Achour (2020). Int. J. Environ. Anal. Chem.. https://doi.org/10.1080/03067319.2019.1662414.
S. H. Borji, S. Nasseri, R. Nabizadeh, A. H. Mahvi, and A. H. Javadi (2011). Iran. J. Health Environ. 3, (4), 369–380.
H. Yuan and J. Xu (2010). Int. J. Chem. Eng. Appl. 1, (3), 214–246.
S. P. Kim, M. Y. Choi, and H. C. Choi (2015). Appl. Surf. Sci. 357, 302–308.
K. Byrappa, A. S. Dayananda, C. P. Sajan, B. Basavalingu, M. B. Shayan, K. Soga, et al. (2008). J Mater Sci. 43, 2348–2355.
Acknowledgements
The authors of this study thank the head of the Central Research Nano Laboratory of Esfarayen University of Technology for the license to use the equipment.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
We declare that we have no financial and personal relationships with other people or organisation that can inappropriately influence our work.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Abbasi, S. Response Surface Methodology for Photo Degradation of Methyl Orange Using Magnetic Nanocomposites Containing Zinc Oxide. J Clust Sci 32, 805–812 (2021). https://doi.org/10.1007/s10876-020-01847-y
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10876-020-01847-y