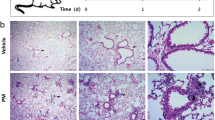
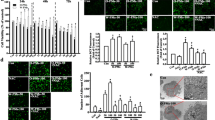
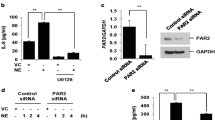
Abstract
Over exposure to particulate matter (PM) could irritate respiratory tract infection; while, Pseudomonas aeruginosa (P. aeruginosa) is one of the main common pathogens. Our study aims are to define whether PM exposure enhances the invasion of P. aeruginosa into the airway epithelia and to characterize the underlying mechanisms. Human bronchial epithelial cells (BEAS-2B) or BEAS-2B transfected by PAFR siRNA were challenged with PM and pretreated with N-acetylcysteine (NAC), LY294002 (PI3K inhibitor), BAY 11-7082 (NF-κB inhibitor), or CV-3988 (PAFR antagonist). P. aeruginosa invasion was evaluated using colony-forming units assay and confocal microscopy. Real-time RT-PCR, immunofluorescence, flow cytometry and western blotting were used to detect the genes or proteins expression. PM exposure promoted P. aeruginosa invasion into BEAS-2B cells through ROS-mediated PI3K pathway which enhanced the expression of PAFR, which could be alleviated by treatment with NAC, LY294002, and BAY 11-7082. Furthermore, NAC and PAFR siRNA attenuated PM-stimulated activation of PI3K pathway. Treatment with PAFR antagonist and siRNA also alleviated PM exposure-induced P. aeruginosa invasion into BEAS-2B cells. Our results demonstrated that PM exposure increased the PAFR expression and activated the PI3K pathway in a ROS-dependent manner. Upregulated PAFR and activated PI3K pathway formed a positive regulatory loop and promoted the invasion of P. aeruginosa into airway epithelia. These mechanisms may provide a novel approach against P.aeruginosa invasion.








Similar content being viewed by others
References
Vijayan VK, Paramesh H, Salvi SS, Dalal AA. Enhancing indoor air quality—the air filter advantage. Lung India. 2015;32(5):473–9. https://doi.org/10.4103/0970-2113.164174.
Neupane B, Jerrett M, Burnett RT, Marrie T, Arain A, Loeb M. Long-term exposure to ambient air pollution and risk of hospitalization with community-acquired pneumonia in older adults. Am J Respir Crit Care Med. 2010;181(1):47–53. https://doi.org/10.1164/rccm.200901-0160OC.
Barnett AG, Williams GM, Schwartz J, Neller AH, Best TL, Petroeschevsky AL, et al. Air pollution and child respiratory health: a case-crossover study in Australia and New Zealand. Am J Respir Crit Care Med. 2005;171(11):1272–8. https://doi.org/10.1164/rccm.200411-1586OC.
Migliaccio CT, Kobos E, King QO, Porter V, Jessop F, Ward T. Adverse effects of wood smoke PM (2.5) exposure on macrophage functions. Inhal Toxicol. 2013;25(2):67–766. https://doi.org/10.3109/08958378.2012.756086.
Mushtaq N, Ezzati M, Hall L, Dickson I, Kirwan M, Png KM, et al. Adhesion of Streptococcus pneumoniae to human airway epithelial cells exposed to urban particulate matter. J Allergy Clin Immunol. 2011;127(5):1236–42.e2. https://doi.org/10.1016/j.jaci.2010.11.039.
Psoter KJ, De Roos AJ, Mayer JD, Kaufman JD, Wakefield J, Rosenfeld M. Fine particulate matter exposure and initial Pseudomonas aeruginosa acquisition in cystic fibrosis. Ann Am Thorac Soc. 2015;12(3):385–91. https://doi.org/10.1513/AnnalsATS.201408-400OC.
Lovewell RR, Patankar YR, Berwin B. Mechanisms of phagocytosis and host clearance of Pseudomonas aeruginosa. Am J Physiol Lung Cell Mol Physiol. 2014;306(7):L591–603. https://doi.org/10.1152/ajplung.00335.2013.
Grigg J. The platelet activating factor receptor: a new anti-infective target in respiratory disease? Thorax. 2012;67(9):840–1. https://doi.org/10.1136/thoraxjnl-2012-202206.
Shukla SD. Platelet-activating factor receptor and signal transduction mechanisms. FASEB J. 1992;6(6):2296–301.
Voss M, Wonnenberg B, Honecker A, Kamyschnikow A, Herr C, Bischoff M, et al. Cigarette smoke-promoted acquisition of bacterial pathogens in the upper respiratory tract leads to enhanced inflammation in mice. Respir Res. 2015;16:41. https://doi.org/10.1186/s12931-015-0204-8.
Zaas DW, Duncan MJ, Li G, Wright JR, Abraham SN. Pseudomonas invasion of type I pneumocytes is dependent on the expression and phosphorylation of caveolin-2. J Biol Chem. 2005;280(6):4864–72. https://doi.org/10.1074/jbc.M411702200.
Wu HM, Wang J, Zhang B, Fang L, Xu K, Liu RY. CpG-ODN promotes phagocytosis and autophagy through JNK/P38 signal pathway in Staphylococcus aureus-stimulated macrophage. Life Sci. 2016;161:51–9. https://doi.org/10.1016/j.lfs.2016.07.016.
Parhamifar L, Andersen H, Moghimi SM. Lactate dehydrogenase assay for assessment of polycation cytotoxicity. Methods Mol Biol (Clifton, NJ). 2013;948:13–22. https://doi.org/10.1007/978-1-62703-140-0_2.
Bai R, Guan L, Zhang W, Xu J, Rui W, Zhang F, et al. Comparative study of the effects of PM1-induced oxidative stress on autophagy and surfactant protein B and C expressions in lung alveolar type II epithelial MLE-12 cells. Biochem Biophys Acta. 2016;1860(12):2782–92. https://doi.org/10.1016/j.bbagen.2016.05.020.
Su R, Jin X, Zhang W, Li Z, Liu X, Ren J. Particulate matter exposure induces the autophagy of macrophages via oxidative stress-mediated PI3K/AKT/mTOR pathway. Chemosphere. 2017;167:444–53. https://doi.org/10.1016/j.chemosphere.2016.10.024.
Barbier M, Oliver A, Rao J, Hanna SL, Goldberg JB, Alberti S. Novel phosphorylcholine-containing protein of Pseudomonas aeruginosa chronic infection isolates interacts with airway epithelial cells. J Infect Dis. 2008;197(3):465–73. https://doi.org/10.1086/525048.
Cundell DR, Gerard NP, Gerard C, Idanpaan-Heikkila I, Tuomanen EI. Streptococcus pneumoniae anchor to activated human cells by the receptor for platelet-activating factor. Nature. 1995;377(6548):435–8. https://doi.org/10.1038/377435a0.
Swords WE, Buscher BA, Ver Steeg Ii K, Preston A, Nichols WA, Weiser JN, et al. Non-typeable Haemophilus influenzae adhere to and invade human bronchial epithelial cells via an interaction of lipooligosaccharide with the PAF receptor. Mol Microbiol. 2000;37(1):13–27.
Deng X, Zhang F, Rui W, Long F, Wang L, Feng Z, et al. PM2.5-induced oxidative stress triggers autophagy in human lung epithelial A549 cells. Toxicol In Vitro. 2013;27(6):1762–70. https://doi.org/10.1016/j.tiv.2013.05.004.
Zhou X, Zhao L, Mao J, Huang J, Chen J. Antioxidant effects of hydrogen sulfide on left ventricular remodeling in smoking rats are mediated via PI3K/Akt-dependent activation of Nrf2. Toxicol Sci. 2015;144(1):197–203. https://doi.org/10.1093/toxsci/kfu272.
Deng X, Rui W, Zhang F, Ding W. PM2.5 induces Nrf2-mediated defense mechanisms against oxidative stress by activating PIK3/AKT signaling pathway in human lung alveolar epithelial A549 cells. Cell Biol Toxicol. 2013;29(3):143–57. https://doi.org/10.1007/s10565-013-9242-5.
Arnout J, van Hecken A, De Lepeleire I, Miyamoto Y, Holmes I, De Schepper P, et al. Effectiveness and tolerability of CV-3988, a selective PAF antagonist, after intravenous administration to man. Br J Clin Pharmacol. 1988;25(4):445–51.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (30772019, 81570028, 81100048), the Scientific Research Foundation for the Returned Overseas Chinese Scholars by State Education Ministry of China, Natural Science Foundation of Shanghai (16ZR1405700), the State Key Development Program of Basic Research of China (973 project) (Grant No: 2015CB553404), Shanghai Municipal Commission of Health and Family Planning (201540078).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Liu, J., Chen, X., Zhou, J. et al. Particulate matter exposure promotes Pseudomonas aeruginosa invasion into airway epithelia by upregulating PAFR via the ROS-mediated PI3K pathway. Human Cell 33, 963–973 (2020). https://doi.org/10.1007/s13577-020-00378-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13577-020-00378-y