Abstract
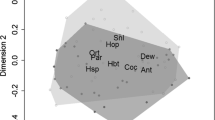
Phytotelmata are habitats that house several species. Oftentimes invertebrate species need to disperse themselves sometimes carried by other animals, an interaction called phoresy. Phoresy can be influenced by different factors, therefore, we aim to investigate which factors influence the phoretic potential between anurans and invertebrates from bromeliads in semideciduous Atlantic Forest, as well as whether there is specificity of invertebrates with any anuran species. We tested if (a) phoresy is more common under conditions with higher rainfall; (b) there are some species of anurans that influence the phoresy of invertebrates more than others; (c) size is an important factor to phoresy. The study was developed during six campaigns in two sites containing the bromeliad Aechmea leptantha. In each campaign, water of bromeliads was collected and all anurans found in bromeliads were processed by washing the skin to collect adhered invertebrates. We recorded the prevalence, average intensity and average abundance of invertebrates between anuran species. We verified the similarity of invertebrates between anuran taxa and we tested the hypothesis of independence between them. In order to identify which factors affect phoresy, we performed a GAMLSS analysis. Invertebrates were present on 54.4% of anurans distributed in five species: Dendropsophus decipiens, Scinax pachycrus, S. x-signatus, S. auratus and Pristimantis ramagii. The estimated densities for prevalence, average intensity and average abundance of invertebrates showed distinct shapes among the anuran species, of which not all participate in the same way in invertebrate transport. The mean phoretic potential showed a significant relationship with anuran length and rainfall.




Similar content being viewed by others
References
Amadeo FE, Dias JD, Segovia BT, Simões NR, Lansac-Tôha FA (2017) Effects of bromeliad flowering event on the community structuring of aquatic insect larvae associated with phytotelmata of Aechmea distichantha Lem. (Bromeliaceae). Acta Limnol Bras. https://doi.org/10.1590/s2179-975x3417
Araújo AP, Bastos CM, Santos RVI, Moura GJB, Melo-Junior M, Tinoco MS (2019) Novel records of phoresy among microcrustaceans and bromeliad treefrogs in the Atlantic Rainforest of Northeast Brazil. Herpetol Notes 12:531–535
Beaty LE, Esser HJ, Miranda R, Norton RA (2013) First report of phoresy by an oribatid mite (Trhypochthoniidae: Archegozetes magnus) on a frog (Leptodactylidae: Engystomops pustulosus). Int J Acarol 39:325–326. https://doi.org/10.1080/01647954.2013.777783
Benjamini Y, Hochberg Y (1995) Controlling the false rate: a practical and powerful approach to multiple testing. R Stat Soc 57:289–300
Benzing DH (2000) Bromeliaceae: profile of an adaptive radiation. Cambridge University Press, Cambridge
Bilder CR, Loughin TM, Nettleton D (2000) Multiple marginal independence testing for pick any/C variables. Commun Stat Simul Comput 29:1285–1316
Bilton DT, Freeland JR, Okamura B (2001) Dispersal in freshwater invertebrates. Annu Rev Ecol Syst 32:159–181. https://doi.org/10.1146/annurev.ecolsys.32.081501.114016
Caceres CE, Soluk DA (2002) Blowing in the wind: a field test of overland dispersal and colonization by aquatic invertebrates. Oecologia 131:402–408. https://doi.org/10.1007/s00442-002-0897-5
Céréghino R et al (2018) Constraints on the functional trait space of aquatic invertebrates in bromeliads. Funct Ecol 32:2435–2447. https://doi.org/10.1111/1365-2435.13141
Conover WJ (1999) Pratical nonparametric statistics. Wiley, Hoboken
Dunn OJ (1961) Multiple comparisons among means. J Am Stat Assoc 56:52–64
Efron B, Tibshirani R (1993) An introduction to the bootstrap. Chapman & Hall/CRC, London
Ferianc O, Lichard M (1967) Birds in the Tribec and Hronsk Inovec Mountains as hosts of tick. Bulletin de L’Organisation Mondiale de la Santé 36:19–23
Frank J, Lounibos L (1987) Phytotelmata: swamps or islands? Fla Entomol 70:14–20
Frank JH, Lounibos LP (2008) Insects and allies associated with bromeliads: a review. Terr Arthropod Rev 1:125–153. https://doi.org/10.1163/187498308X414742
Givnish TJ et al (2011) Phylogeny, adaptive radiation, and historical biogeography in Bromeliaceae: insights from an eight-locus plastid phylogeny. Am J Bot 98:872–895. https://doi.org/10.3732/ajb.1000059
Houck M, O’Connor B (1991) Ecological and evolutionary significance of phoresy in the astigmata. Annu Rev Entomol 36:611–636
Jocque M, Fiers F, Romero M, Martens K (2013) Crustacea in phytotelmata: a global overview. J Crustacean Biol 33:451–460
Kabir MT, Snow JW (2019) Phoresy or an accident? Trafficking of flower-feeding thrips by pollen foraging bees. Ecology 100:01–03
Legendre P, Legendre L (2012) Numerical ecology. Elsevier, Amsterdam
Lindén A, Mäntyniemi S (2011) Using the negative binomial distribution to model overdispersion in ecological count data. Ecology 92:1414–1421
Lopez LCS, Filizola B, Deiss I, Rios RI (2005) Phoretic behaviour of bromeliad annelids (Dero) and Ostracods (Elpidium) using frogs and lizards as dispersal vectors. Hydrobiologia 549:15–22. https://doi.org/10.1007/s10750-005-1701-4
Lopez LCS, Rodrigues PJP, Rl R (1999) frogs and snakes as phoretic dispersal agents of bromeliad ostracods (Limnocytheridae: Elpidium) and annelids (Naididae: Dero). Biotropica 31:705–708
Lyra TL (1976) Microflora de bromeliaceas do estado de Pernambuco. Brasil Memórias do Instituto Oswaldo Cruz 74:37–50
Maaten L, Hinton G (2008) Visualizing data using t-SNE. J Mach Learn Res 9:2579–2605
Macchioni F, Magi M, Mancianti F, Perrucci S (2005) Phoretic association of mites and mallophaga with the pigeon fly Pseudolynchia canariensis. Parasite 12:277–279
Maguire B (1963) The passive dispersal of small aquatic organisms and their colonization of isolated bodies of water. Ecol Monogr 33:161–185
Maguire B (1971) Phytotelmata biota and community structure determination in plant-held waters. Annu Rev Ecol Evol Syst 2:439–464
Moroti MT, Muscat E, Pedrozo M, Machado IF, Sabagh LT, Santana DJ (2019) Interaction between ostracods and anurans:a review and new records in Brazil. Phyllomedusa 18:269–275
Mattos TM, Carvalho DR, de Brito MS, Araújo FG (2018) Occurrence of phoresy between Ancistrus multispinis (Actinopterygii: Siluriformes) and Ichthyocladius sp. (Diptera: Chironomidae) in Atlantic forest streams. Southeast Brazil Zool 35:1–6. https://doi.org/10.3897/zoologia.35.e13255
Mogi M (2004) Phytotelmata: hidden freshwater habitats supporting unique faunas. Freshwater. In: Yule CM, Yong HS (eds) Freshwater invertebrates of the Malaysian Region. National Academy of Sciences Malaysia, Kuala Lumpur, p 861
Muzzall PM (1991) Helminth infracommunties of the frogs Rana catesbeiana and Rana clamitans from Turkey Marsh, Michigan. J Parasitol 77:366–371
Paula Júnior ATd, Rosa BFJV, Alves RG, Divino AC (2017) Aquatic invertebrates associated with bromeliads in Atlantic Forest fragments. Biota Neotrop. https://doi.org/10.1590/1676-0611-bn-2016-0188
Peixoto OL (1995) Associação de anuros a bromeliáceas na Mata Atlântica. Rev Univ Rural Sér Ciênc da Vida 17:75–83
R Core Team (2019) R: a language and environment for statistical computing Vienna, Austria. URL https://www.R-project.org/. Accessed 22 May 2020
Reynolds DR, Reynolds AM, Chapman JW (2014) Non-volant modes of migration in terrestrial arthropods. Rev Artic 2:8–28
Rocha CFD, Cogliatti-Carvalho L, Nunes-Freitas AF, Rocha-Pessôa TC, Dias AdS, Ariani CV, Morgado LN (2004) Conservando uma larga porção da diversidade biologica através da conservação Bromeliaceae. Vidalia 2:52–72
Rubin DB (1981) The bayesian bootstrap. Ann Stat 9:130–134
Sabagh LT, Dias RJP, Branco CWC, Rocha CFD (2011) News records of phoresy and hyperphoresy among treefrogs, ostracods, and ciliates in bromeliad of Atlantic forest. Biodivers Conserv 20:1837–1841. https://doi.org/10.1007/s10531-011-0050-z
Sabagh LT, Rocha CFD (2014) Bromeliad treefrogs as phoretic hosts of ostracods. Naturwissenschaften 101:493–497. https://doi.org/10.1007/s00114-014-1178-y
Sabagh LT, Ferreira RB, Rocha CFD (2017) Host bromeliads and their associated frog species: further considerations on the importance of species interactions for conservation. Symbiosis 73:201–211. https://doi.org/10.1007/s13199-017-0500-9
Saul-Gershenz LS, Millar JG (2006) Phoretic nest parasites use sexual deception to obtain transport to their host's nest. Proc Natl Acad Sci USA 103:14039–14044. https://doi.org/10.1073/pnas.0603901103
Sládeèek V (1983) Rotifers as indicators of water quality. Hydrobiologia 100:169–201
Stark T, Brouwer D, Ploeg R, Lenders T (2017) First record of phoresy or possible parasitism by the fresh water leech Helobdella stagnalis (Glossiphoniidae) on Lissotriton helveticus (Caudata: Salamandridae) in the Netherlands. Herpetol Notes 10:717–719
Stasinopoulos DM, Rigby RA (2007) Generalized Additive Models for Location Scale and Shape (GAMLSS). R J Stat Softw 23:1–46
Tukey JW (1977) Exploratory data analysis. Addison-Wesley Publishing Company, Boston
Toledo GM, Morais DH, Silva RJ, Anjos LA (2013) Helminth communites of Leptodactylus latrans (Anura: Leptodactylidae) from the Atlantic rainforest, south-eastern Brazil. J Helminthol. https://doi.org/10.1017/S0022149X1300076X
Ulloa Ulloa C et al (2017) An integrated assessment of the vascular plant species of the Americas. Science 358:1614–1617. https://doi.org/10.1126/science.aao0398
Waleckx E, Montalvo-Balam TdJ, Pinzón-Canul A, Arnal A, Marti G, Martínez PA (2018) First report of phoresy by an oribatid mite (Acari: Oribatida) on a triatomine bug (Hemiptera: Reduviidae). Int J Acarol 44:210–211. https://doi.org/10.1080/01647954.2018.1487467
Acknowledgements
The authors would like to thank the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – Brazil (CAPES) – Finance Code 001, for the PROAP financial assistance and the Masters’ fellowship granted to the first author, and the Chico Mendes Institute for Biodiversity Conservation (ICMBio), for permits to research (licence: ICMBio 57241–1).
Author information
Authors and Affiliations
Contributions
APA, MMJ, GJBM and MST conceived the project, reviewed and approved the final draft; APA and APD carried out data collection in the field and laboratory; AHCM analyzed the data, prepared the figures and tables, reviewed and approved the final article; APA and AHCM wrote the manuscript; MMJ, GJBM and MST contributed reagents/materials/analysis tools.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
de Araújo, A.P., Marques, A.H.C., Dantas, A.P. et al. Assisted phoresy of invertebrates by anurans in tank bromeliads: interspecific relationship. Aquat Sci 82, 64 (2020). https://doi.org/10.1007/s00027-020-00732-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00027-020-00732-0