Abstract
There are no practical and valid methods for the assessment of individualised physical activity (PA) intensity in observational studies. Therefore, we investigated the validity of commonly used metabolic equivalent of tasks (METs) and pre-determined PA intensity classification methods against individualised PA intensity classification in 35 children 7–11-years-of-age. Then, we studied validity of mean amplitude deviation (MAD) measured by accelerometry during self-paced walking and running in assessment of individualised PA intensity. Individualised moderate PA (MPA) was defined as V̇O2 ≥ 40% of V̇O2reserve and V̇O2 < ventilatory threshold (VT) and vigorous PA (VPA) as V̇O2 ≥ VT. We classified > 3–6 (or alternatively > 4–7) METs as MPA and > 6 (> 7) METs as VPA. Task intensities were classified according to previous calibration studies. MET-categories correctly identified 25.9–83.3% of light PA, 85.9–90.3% of MPA, and 56.7–82.2% of VPA. Task-specific categories correctly classified 53.7% of light PA, 90.6% of MPA, and 57.8% of VPA. MAD during self-paced walking discriminated MVPA from light PA (sensitivity = 67.4, specificity = 88.0) and MAD during self-paced running discriminated VPA from MPA (sensitivity = 78.8, specificity = 79.3). In conclusion, commonly used methods may misclassify PA intensity in children. MAD during self-paced running may provide a novel and practical method for determining individualised VPA intensity in children.
Similar content being viewed by others
Introduction
Free-living physical activity (PA) has been inversely associated with cardiometabolic risk in children1. Moreover, higher PA intensity may confer greater cardiometabolic health benefits than lower PA intensity2,3,4. Therefore, accurate assessment of PA intensity is important for informing health-related PA recommendations and in research on dose–response relationships. Nowadays, volume and intensity of PA in observational studies are typically assessed using accelerometers5,6. However, previous studies have used fixed acceleration magnitude cut-offs to define different PA intensities without accounting for individual variation in exercise capacity6. The continuing use of fixed cut-offs may have obscured our understanding on the prevalence of children accumulating recommended 60 min of daily moderate-to-vigorous PA (MVPA) and the role of PA intensity in health outcomes among youth7,8.
Fixed acceleration intensity cut-offs based on a single absolute acceleration magnitude value have been found to underestimate PA volume and intensity in lower fit and overweight adults8. Furthermore, metabolic equivalent of task (MET) is commonly used to assess PA intensity9 and to calibrate accelerometry cut-offs10. MET approach has been criticised because METs are confounded by body composition and METs also underestimate PA intensity in overweight and obese individuals9. In addition, previous calibration studies in children have provided several different cut-offs for light (LPA), moderate (MPA), and vigorous PA (VPA) leading to a large variation in the proportion of children meeting the PA recommendations7,11,12. However, some evidence suggests that of fixed acceleration magnitude cut-offs, those provided by Evenson et al.13 and Freedson et al.14 may be the most suitable for estimating PA intensity in children15. Nevertheless, these studies have subjectively decided calibration task intensities to determine LPA, MPA, and VPA irrespective of their true metabolic cost7,11,12. Therefore, previous studies do not have a strong physiological rationale for the suggested PA intensity cut-offs.
Expressing PA intensity relative to cardiorespiratory fitness (CRF) have been shown to reduce differences in PA volume and intensity between normal and overweigh adults and between adults with varying levels of CRF8. Therefore, some studies have recommended that PA intensity should be related to individualised oxygen uptake (V̇O2) reserve to account for the individual variation in CRF and the inability of fixed cut-offs to capture individualised PA intensity16. Therefore, defining PA intensity as a percentage of V̇O2 reserve provides more appropriate estimate of individualised PA intensity than fixed PA intensity cut-offs. However, because of individual variation in ventilatory threshold (VT), metabolic responses can differ significantly between individuals who exercise at the same proportion of their V̇O2 reserve17,18. PA performed below VT, a non-invasive equivalent of lactate threshold, has been considered as MPA that can be maintained prolonged periods without constantly increasing blood lactate concentration19. Furthermore, PA above VT leads to an increased lactate concentration and exhaustion and is often defined as VPA19. Exercise training intensity based on VT has also been shown to trigger larger physiological adaptations and to reduce individual variation in physiological responses to exercise compared to exercise training intensity based on percentage of V̇O2 reserve20,21 suggesting the validity of VT in the exercise prescription and calibration of VPA. Furthermore, some evidence in adults suggests that calibrating PA intensity cut-offs using lactate threshold or VT provides more accurate estimates of VPA than fixed cut-offs based on METs22. However, there are no previous studies utilising VT in calibration of accelerometry cut-offs for VPA in children.
Individual calibration of cut-offs may provide superior classification accuracy for LPA, MPA, and VPA in children23. Some studies have calibrated accelerometry cut-offs in a subsample of a larger study population and created fixed sample specific PA intensity cut-offs24 or used individually determined intensity cut-offs based on optimum walking speed and transition from walking to running during an incremental cardiopulmonary exercise test on a treadmill25. However, the individualised calibration of accelerometry cut-offs against true metabolic cost of activity or optimum walking speed and transition from walking to running during an incremental cardiopulmonary exercise test is not always feasible, because of their time consuming and resource intensive nature. On the other hand, self-selected walking speed has been found to reflect individualised MPA intensity26, and we hypothesised that self-preferred walking speed may be a feasible method to individually calibrate MPA. However, there are no studies on the validity of self-paced walking and running in the individual calibration of accelerometry cut-offs against V̇O2 reserve and VT in children.
To improve our understanding on the role of PA intensity it is essential to investigate the validity of commonly used methods used to define PA intensity and to investigate novel methods aiming to improve PA intensity estimation in children. Therefore, we first investigated the validity of PA intensity cut-offs based on METs and task-specific calibration activities against individualised PA intensity cut-offs in children. Second, we investigated whether acceleration mean amplitude deviation (MAD) normalised for MAD measured during self-paced walking and running provide better classification accuracy for individualised PA intensity than fixed MAD cut-offs.
Results
Characteristics of participants and different physical activities
Characteristics of the participants are shown in Table 1. METs, absolute MAD, V̇O2 as a % of V̇O2 reserve, V̇O2 as a % of V̇O2 at VT, MAD relative to MAD during self-paced walking and running, and V̇O2 normalised for skeletal muscle mass (SMM) or body mass (BM) increased with increasing treadmill speed and were higher during self-paced running than during self-paced walking (p < 0.001 for main effect for all comparisons, Fig. 1). MAD did not differ between walking or running on a treadmill for 6 km/h and playing hopscotch (p = 0.126). METs, V̇O2 as a % of V̇O2 reserve, V̇O2 as a % of V̇O2 at VT, and V̇O2 normalised for SMM did no differ statistically significantly between walking up and down the stairs and playing hopscotch (p = 0.059 to 0.106).
Differences in METs, MAD, V̇O2 as a % of V̇O2 reserve, V̇O2 as a % of V̇O2 at VT, and V̇O2 normalised for skeletal muscle mass (SMM) or body mass (BM) in different physical activities. 1: walking on a treadmill 4 km/h, 2: walking or running on a treadmill 6 km/h, 3: walking or running on a treadmill 8 km/h, 4: walking up and down the stairs, 5: playing hopscotch, 6: self-paced walking, 7: self-paced running. MET metabolic equivalent of task, MAD mean amplitude deviation, V̇O2 oxygen uptake, VT ventilatory threshold.
Validity of PA intensity based on MET-classification and task-specific classification against individualised PA intensity classification
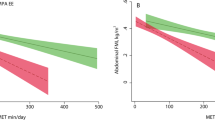
Compared to individualised PA intensity classification, MET-classification correctly classified 25.9% of LPA, 85.9% of MPA, and 82.2% of VPA (χ2(4) = 151, p < 0.001, Cramer’s V = 0.6, Fig. 2A,C,D). When we used > 4–7 METs to define MPA and > 7 METs to define VPA, 83.3% of LPA, 90.3% of MPA, and 56.7% of VPA (χ2(4) = 151, p < 0.001, Cramer’s V = 0.704) were correctly classified. Task-specific PA intensity classification correctly classified 53.7% of LPA, 90.6% of MPA, and 57.8% of VPA (χ2(4) = 147.3, p < 0.001, Cramer’s V = 0.595). Previously published fixed MAD cut-offs correctly classified 76.9% of LPA, 52.5% of MPA, and 54.4% of VPA (χ2(4) = 88.5, p < 0.001, Cramer’s V = 0.47, Fig. 2B).
Agreement between individualised physical activity intensity categories based on V̇O2 reserve and ventilatory threshold and fixed physical activity intensity categories based on metabolic equivalent of tasks (METs) and mean amplitude deviations (MADs). (A) Individualised physical activity intensity versus METs. (B) Individualised physical activity intensity versus MADs, (C) MET-based physical activity intensity categories versus V̇O2 as % of V̇O2 reserve, (D) MET-based physical activity intensity categories versus V̇O2 as % of V̇O2 at ventilatory threshold. LPA individualised light physical activity (V̇O2 < 40% of V̇O2 reserve), MPA individualised moderate physical activity (V̇O2 ≥ 40% of VO2 reserve but < V̇O2 at VT), VPA individualised vigorous physical activity = ≥ VO2 at VT). ≤ 3 METs = light physical activity, > 3–6 METs = moderate physical activity, > 6 METs = vigorous physical activity.
Physical activity intensity during self-paced walking and running
The mean of V̇O2 as a % of V̇O2 of reserve was 37.4% (min 19.3, max 54.1, SD = 8.7) and the median of V̇O2 as a % of V̇O2 at VT was 68.5% (min 41, max 133, IQR = 16.1) during self-paced walking (Fig. 1). Self-paced walking was categorised as LPA in 55.2% (N = 16), MPA in 34.5% (N = 10), and VPA in 10.3% (N = 3) of the children based on individualised PA intensity classification. Mean MET value during self-paced walking was 4.1 (min 2.5, max 6.8, SD = 1.0).
The median of V̇O2 as a % of V̇O2 on reserve was 100% (min 54.8, max 100, IQR = 10.9) and V̇O2 as a % of V̇O2 at VT was 152.5% (min 90.2, max 229.6, IQR = 29.1) during self-paced running. Self-paced running was MPA for 3.8% (N = 1) and VPA for 96.2% (N = 25) of the children. The median MET value during self-paced running was 8.5 (min 6.4, max 12.7, IQR = 1.4).
MAD during self-paced walking and running in classifying individualised physical activity intensity
MAD as a % of MAD during self-paced walking was able to discriminate LPA from MVPA in 67.4% of the cases (Table 2). MAD as a % of MAD during self-paced running were able to discriminate VPA from MPA in 78.8% of the cases. Fixed MAD thresholds were able to discriminate LPA from MVPA in 65.8% of the cases and VPA from MPA in 66.7% of the cases. The ability to correctly identify individualised PA intensity increased when walking up and down the stairs and playing hopscotch were excluded from the data.
The ability of MAD as a % of MAD during self-paced walking and running and absolute MAD to discriminate PA intensities using different PA intensity classifications is presented in Table 2. Notably, MAD during self-paced walking and absolute MAD had higher sensitivity and specificity to correctly classify PA intensity based on task-specific calibration tasks compared to other methods used to classify PA intensity.
Discussion
We found that defining PA intensity using fixed METs and task-specific classification methods may lead to large errors in the classification of individualised PA intensity in children. We also found that MAD values measured during different laboratory activities normalised to MAD values measured during self-paced running had acceptable sensitivity to discriminate individualised VPA from MPA. Furthermore, we showed that MAD values measured during different laboratory activities normalised to MAD during self-paced running was more accurate in discriminating VPA from MPA than absolute MAD especially when more complex and intermittent physical activities, such as walking up and down the stairs and playing hopscotch, were included in the analyses.
We observed that METs, task-specific calibration, or previous MAD cut-offs27 misclassified LPA in up to 70% and VPA in 20–45% of the cases. Furthermore, compared with 3 METs as a cut-off for MVPA and 6 METs as a cut-off for VPA, using > 4 METs and > 7 METs improved the classification accuracy to discriminate LPA from MVPA, but decreased the classification accuracy to differentiate VPA from MPA. These findings are in line with the observations in adults showing that fixed PA intensity cut-offs based on METs can lead to a significant misclassification of PA intensity and underestimate true intensity and volume of PA especially in overweight or unfit individuals8. To the best of our knowledge, similar findings have not been published in children. None of the previous studies utilising direct measurements of V̇O2 have anchored proposed PA intensity cut-offs on physiological thresholds based on V̇O2 data or took individual differences in V̇O2peak into account13,28,29. Furthermore, we found only small difference in MAD-based fixed PA intensity cut-offs for MPA (0.062 g) and VPA (0.0583) between our study and the study by Aittasalo et al. using comparable task-specific calibration method27 suggesting that task-specific PA intensity calibration has relatively good agreement between samples, but the cut-offs do not accurately reflect true PA intensity. These findings together indicate that common methods used to classify PA intensity may cause remarkable bias in the assessment of individualised PA intensity in children.
We observed that MAD measured during different activities normalised for MAD measured during self-paced walking or running were able to discriminate MVPA and VPA, but the sensitivity of those measures to correctly classify PA intensity was lower than in previous studies13,30. Differences in the methods used to classify PA intensity most likely explain these differences. Most previous studies providing individualised25 or population specific13,27,30,31 fixed accelerometry cut-offs have used predetermined task-specific activities or evaluated the validity of the cut-offs using fixed MET-thresholds. We showed that the sensitivity and specificity of absolute MAD to discriminate task-specific PA intensity was above 80% but the ability of correctly classify individualised PA intensity was below 70%. Another reason for the discrepancy in classification accuracy with previous studies may be that our study included activities with low acceleration but high energetic demand, such as walking up and down the stairs which occurs frequently in free living, while physical activities in some previous studies have been simple ambulatory activities30,31. Therefore, our findings suggest that fixed accelerometry cut-offs have limited validity in the assessment of individualised PA intensity.
MAD relative to MAD during self-paced running had better sensitivity to discriminate VPA from LPA and MPA compared with fixed MAD cut-off. However, the classification accuracy of MAD during self-paced running and fixed MAD cut-offs was comparable when the data included only walking and running activities. Nevertheless, using only activities including walking and running in the calibration of PA intensity cut-offs may not reflect habitual physical activities in children and would lead to errors in the estimation of habitual PA intensity. All children, except one, operated at intensity above VT during self-paced running suggesting that anchoring VPA to MAD values measured during self-paced running reflects VPA in almost all children. Fixed MAD cut-off for VPA based on our sample and from previous samples27 underestimated PA intensity in 33–46% of the cases while cut-offs based on MAD as a % of MAD during self-paced running misclassified 21% of the cases. Therefore, our results suggest that MAD during self-paced running provides better estimate of VPA in children than fixed MAD cut-offs.
Against our hypotheses, our results do not support the superiority of MAD during self-paced walking in the individual calibration of MVPA in children. Relatively large variation between children in V̇O2 during self-paced walking varying from less than 20% up to 54% of V̇O2 reserve may partly explain our observation. Differences in neuromuscular maturation, which may have an effect on ability to control walking intensity and walking economy, may explain this large variation in V̇O2 during self-paced walking in children aged 7–11 years32.
The strengths of the present study include a valid and simultaneous assessment of V̇O2 and accelerometry during different activities, assessment of true resting V̇O2, V̇O2peak and VT, and the use of individualised PA intensity in categorising LPA, MPA, and VPA. Our sample was also relatively representative to general Finnish population regarding V̇O2peak33. We also had variable physical activities mimicking normal daily activities performed by the children. It would have been optimal to include free play and tasks where participants would have been allowed to perform free tasks in their normal environments to increase the ecological validity of the study. V̇O2peak and VT were assessed during a maximal cycle ergometer test and V̇O2peak was adjusted using the data from the treadmill running or self-paced running if higher V̇O2 was observed during those activities. Therefore, it is possible that we have underestimated true V̇O2max in some participants. A maximal treadmill exercise test with a supramaximal validation test would have needed to measure true V̇O2max to optimally reflect ambulatory activities in the present study. Furthermore, we defined MVPA as V̇O2 ≥ 40% of V̇O2 reserve. Although several previous studies have also defined MVPA as 40–55% of V̇O2peak5, the physiological rationale for these limits to separate LPA from MVPA is lacking. Therefore, the classification accuracy of self-paced walking could have been different with another individulised intensity threshold. More research is warranted to fully understand PA intensity in children. Finally, although children were required to arrive at fasted state to the resting measurement, they were not asked to avoid all exercise a day before the resting measurements. Therefore, exercise during the previous day could have had some minor effect on the resting V̇O2 in the present study. However, children have been found to recover fast even from strenuous exercise34.
In conclusion, our results suggest that fixed MET-values or predetermined task-specific calibration activities should be used with caution in the classification of PA intensity or in the calibration of accelerometry cut-offs in children. Moreover, child-specific MAD values corresponding approximately 50% of the MAD values measured during self-paced running may provide more accurate, feasible, and practical method for individual calibration of VPA in children. Furthermore, our results suggest that fixed MAD cut-offs may be acceptable if children only walk and run, but not when they perform more complex and intermittent activities. Therefore, individualised MAD values may provide better estimates of VPA in real life setting. Further studies investigating the physiological responses to different exercise intensities and whether self-paced or optimum walking speed25, self-paced running, or the transition from walking to running can be used in individual calibration of PA intensity in large-scale observational studies in children are warranted. Finally, there is a need for further studies investigating how individualised PA volume and intensity are related to health and wellbeing in children.
Methods
Participants
This study was based on the laboratory phase of the Children’s Physical Activity Spectrum (CHIPASE) study35. A total of 35 children (21 girls, 14 boys) aged 7–11 years were recruited from local schools and volunteered to participate in the study. Children were included if they were apparently healthy and were able to perform the physical activities at moderate and vigorous intensities. Children with chronic conditions or disabilities were excluded from the study. The study protocol was approved by the Ethics Committee of the University of Jyväskylä. All children gave their assents and their parents/caregivers gave their written informed consents. The study was conducted in agreement with the Declaration of Helsinki.
Study protocol
The participants visited the laboratory three times. At the first visit, research staff explained the research protocol to children and their parents. They were also familiarised to the laboratory environment and the measurement equipment. At the second visit, children arrived at the laboratory in the morning after 10–12 h overnight fast for the assessment of anthropometrics, body composition, and resting V̇O2. At the third visit, children were asked to perform following activities for 4.5 min in a random order interspersed with 1-min rest: sitting quietly, sitting while playing a mobile game, standing quietly, standing while playing a mobile game, playing hopscotch, walking up and down the stairs, and walking or running on a treadmill at 4, 6, and 8 km/h. They were also asked to walk and run around an indoor track at self-chosen speed for 4.5 min. At the end of the third visit, children performed maximal cardiopulmonary exercise test on a bicycle ergometer.
Assessments
Body size and body composition
Stature was measured to the nearest 0.1 cm using a wall-mounted stadiometer. BM, SMM, fat mass, fat free mass, and body fat percent were measured by InBody 770 bioelectrical impedance device (Biospace Ltd., Seoul, Korea). Body mass index (BMI) was calculated by dividing body weight with body height squared and body mass index standard deviation score (BMI-SDS) was computed using the Finnish references36.
Oxygen uptake during rest and different physical activities
Mobile metabolic cart (Oxycon mobile, CareFusion Corp, USA) was calibrated and dead space was adjusted to 78 ml for the petite size of the face mask following the manufacturer’s recommendations. V̇O2, carbon dioxide production (V̇CO2) and respiratory exchange ratio (RER) were collected breath by breath and computed in non-overlapping 1 s epoch lengths. Resting V̇O2 was measured in the quiet room while a child lying down as still as possible watching an age-appropriate cartoons. Resting V̇O2 was determined as the mean value between the 15th and 25th minute of 30 min of supine rest when the steady state was reached37. When steady stated was not observed or there were abnormal spikes in the data between 15 and 25th minute, we visually determined the steady state from the whole measurement period for further analysis. The data were visually checked among six participants and the changes made in analysis window varied between 50 s and five minutes. In physical activities, V̇O2 was averaged over 2 min from the 3rd and 4th minutes of each task when the plateau in V̇O2 and V̇CO2 was observed38. Although the order of physical activities was randomised, we also confirmed that V̇O2 returned near to baseline levels during the 1-min rest (Supplementary figure 1).
Peak oxygen uptake and oxygen uptake at ventilatory threshold
Cardiorespiratory fitness was assessed by a maximal ramp exercise test on an electromagnetically braked Ergoselect 200 K electromagnetic cycle ergometer (Ergoline, Bitz, Germany)33. The protocol included 2-min resting period sitting on an ergometer, a 3-min warm-up with a workload of 20 W, and an incremental exercise period with increase of workload either by 1 W/3 s (totalling 20 W/minute for children > 150 cm), 1 W/4 s (totalling 15 W/minute for children 126–150 cm), or 1 W/6 s (totalling 10 W/minute for children ≤ 125 cm) until voluntary exhaustion39. The participants were asked to keep the cadence at 70–80 during the test. The test was terminated when the participant was unable to keep the cadence of 65 or required to stop. Participants were verbally encouraged to exercise until voluntary exhaustion.
Respiratory gas exchange was assessed directly by breadth-by-breadth method using the metabolic cart from the 2-min resting period sitting on the ergometer until the voluntary exhaustion and were averaged over 15-s periods. We defined peak cardiorespiratory capacity as the highest V̇O2 achieved in the exercise test (V̇O2peak) averaged over 15 s recorded during the last minute of the exercise test and normalised it for SMM. If higher V̇O2 was observed during running on a treadmill for 8 km/h or during self-paced running (N = 21) than during the maximal cycle exercise tests (N = 14), the higher V̇O2 value was used as a measure of V̇O2peak. Beat-by-beat heart rate (HR) was continuously recorded during the exercise test using Polar H7 HR sensor (Polar Electro, Kempele, Finland).
The cardiopulmonary exercise test was considered maximal if the primary and secondary objective and subjective criteria indicated maximal effort and maximal cardiorespiratory capacity (a plateau of V̇O2 regardless of increasing workload, HR > 85% of predicted, respiratory exchange ratio > 1.00, or flushing and sweating), and the exercise physiologist supervising the exercise test considered the test maximal40.
V̇O2 at VT was individually determined by two exercise physiologists using modified V-slope method41 and any disagreements were solved by these two exercise physiologists. The VT was identified as a time point where the increase in V̇CO2 was steeper than the increase in V̇O2 during the maximal cardiopulmonary exercise test on a cycle ergometer. In determination of VT, we used data averaged over 15 seconds41. V̇O2 at VT was verified utilising the equivalents for V̇E/V̇CO2 and V̇E/V̇O2. According to equivalent method V̇O2 at VT was defined as a rate of V̇O2 where V̇E/V̇O2 begins to increase without an increase in V̇E/V̇CO2.
Accelerometry during different physical activities
Movement was measured by triaxial accelerometer (X6-1a, Gulf Coast Data Concepts Inc., Waveland, USA). We used raw acceleration data in actual g-units with the high range up to 6 g with 16-bit A/D conversion, and sampling at 40 Hz. The resultant acceleration of the triaxial accelerometer signal was calculated from \(\sqrt{{x}^{2}+{y}^{2}+{z}^{2}}\), where x, y and z were the measurement sample of the raw acceleration signal in x-, y-, and z-directions. The X6-1a accelerometer has been shown to produce congruent results with the ActiGraph GT3X accelerometer in children42. The MAD was calculated from the resultant acceleration in non-overlapping 1 s epoch. MAD describes the mean distance of data points around the mean ( \(\frac{1}{n}\sum_{i=1}^{n}|{r}_{i -}\stackrel{-}{r}|\) where n was the number of samples in the epoch, \({r}_{i}\) is the ith resultant sample within the epoch and \(\stackrel{-}{r}\) is the mean resultant value of the epoch)27,43. The mean of the 1 s MAD values (g) were calculated in the 2 min time epochs for each activity and in 10 min epoch for lying down and were reported as the outcomes. MAD measured during different activities as a percentage of MAD measured during self-paced walking or running was computed as (MADactivity/MADself-paced walking or running) * 100. To obtain individualised child-specific MAD values representing the cut-offs for MPA and VPA, the absolute child-specific MAD value measured during self-paced walking or self-paced running should be used and the appropriate multiplier provided in the Table 2 should be applied.
Definitions of intensity in different physical activities
Individualised intensity classification. We defined individualised PA intensity using V̇O2 reserve and V̇O2 at VT. V̇O2 reserve was calculated as (V̇O2 during PA task/V̇O2peak—V̇O2 during rest) × 100. LPA was defined as V̇O2 below 40% and MPA as V̇O2 ≥ 40% of V̇O2 reserve16 to < V̇O2 at VT19. VPA was defined as V̇O2 at or above V̇O2 at VT19. MVPA was defined as activity V̇O2 ≥ 40% of V̇O2 reserve16.
MET-based intensity classification. MET values were computed as V̇O2 measured during the physical activities/V̇O2 during supine rest. PA intensity during different tasks was categorised as follows: LPA was defined as > 1.5–3 METs, MPA as > 3–6 METs, and VPA as > 6 METs3. We also performed additional analyses using the cut-offs of > 4 METs for MPA and > 7 METs for VPA. MVPA was defined as > 3 or > 4 METs.
Predetermined task-specific intensity classification. We classified walking on a treadmill for 4 km/h as LPA, running on treadmill for 6 km/h, walking up and down the stairs playing hopscotch, and walking around an indoor track on self-chosen speed as MPA, and running on a treadmill for 8 km/h and running around an indoor track on self-chosen speed as VPA according to previous calibration studies7,11,12. MVPA was then classified as tasks excluding tasks considered LPA. We also investigated the agreement of previously published fixed MAD cut-offs for LPA (< 332 mg), MPA (332 mg), and VPA (558.3 mg) based on predetermined task-specific intensity classification provided by Aittasalo et al.27 with individualised PA intensity.
Statistical methods
Basic characteristics between girls and boys were compared using Student’s t-test for normally distributed continuous variables and Mann–Whitney U-test for skewed continuous variables. We investigated differences in METs, absolute MAD, V̇O2 as a % of V̇O2 reserve, V̇O2 as a % of V̇O2 at VT, MAD relative to MAD during self-paced walking and running, and V̇O2 normalised for SMM or BM in different physical activities using mixed-effects repeated measures ANOVA. We investigated the validity of MET-based PA intensity classification, task-specific intensity classification, and previously published fixed MAD27 cut-offs against individualised PA intensity cut-offs using χ2 test and Cramer’s V.
We used receiver operating characteristics (ROC) curves to investigate the optimal cut-off for MAD as a % of MAD during self-paced walking or running and absolute MAD to differentiate LPA from MVPA and LPA and MPA from VPA using individualised PA intensity classification. The area under the curve (AUC) is considered a measure of the effectiveness of the predictor variable to correctly discriminate MVPA from LPA and VPA from MPA (sensitivity) and to correctly discriminate LPA from MVPA and MPA from VPA (specificity). An AUC of 1 represents the ability to perfectly identify MVPA or VPA from other intensities, whereas an AUC of 0.5 indicates no greater predictive ability than chance alone. The optimal cutoff was determined by the Youden index44, which is the maximum value of J that is computed as: sensitivity + specificity − 1.
Student´s t-test, the Mann–Whitney U-test, and the χ2 test were performed using the SPSS Statistics, Version 23.0 (IBM Corp., Armonk, NY, USA). The data were visualised and the mixed-effects repeated measures ANOVA were performed by the GraphPad Prism, version 8.0.2 (Graph Pad Software, Inc., San Diego, CA, USA). The ROC curve analyses were performed using MedCalc Statistical Software, Version 16.1 (MedCalc Software bvba, Ostend, Belgium).
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Poitras, V. J. et al. Systematic review of the relationships between objectively measured physical activity and health indicators in school-aged children and youth. Appl. Physiol. Nutr. Metab. 41, 197–239 (2016).
Väistö, J. et al. Longitudinal associations of physical activity and sedentary time with cardiometabolic risk factors in children. Scand. J. Med. Sci. Sports 29, 113–123 (2019).
Collings, P. J. et al. Cross-sectional associations of objectively-measured physical activity and sedentary time with body composition and cardiorespiratory fitness in mid-childhood: The PANIC Study. Sport. Med. 47, 769–780 (2017).
Moore, J. B. et al. Associations of vigorous-intensity physical activity with biomarkers in youth. Med. Sci. Sports Exerc. 49, 1366–1374 (2017).
Corder, K., Ekelund, U., Steele, R. M., Wareham, N. J. & Brage, S. Assessment of physical activity in youth. J. Appl. Physiol. 105, 977–987 (2008).
Strath, S. J. et al. Guide to the assessment of physical activity: Clinical and research applications: a scientific statement from the American Heart Association. Circulation 128, 2259–2279 (2013).
Migueles, J. H. et al. Comparability of published cut-points for the assessment of physical activity: Implications for data harmonization. Scand. J. Med. Sci. Sports 29, 566–574 (2019).
Kujala, U. M. et al. Physical activity: absolute intensity versus relative-to-fitness-level volumes. Med. Sci. Sports Exerc. 49, 474–481 (2017).
Tompuri, T. T. Metabolic equivalents of task are confounded by adiposity, which disturbs objective measurement of physical activity. Front. Physiol. 6, 1–6 (2015).
Vähä-Ypyä, H. et al. Validation of cut-points for evaluating the intensity of physical activity with accelerometry-based Mean Amplitude Deviation (MAD). PLoS ONE 10, 1–13 (2015).
Ravagnani, F. et al. Application of the rosetta stone to understand how much MVPA preschoolers accumulate: A systematic review. J. Sci. Med. Sport 20, 849–855 (2017).
Gao, Y. et al. Children’s physical activity and sedentary time compared using assessments of accelerometry counts and muscle activity level. PeerJ 6, e5437 (2018).
Evenson, K. R., Catellier, D. J., Gill, K., Ondrak, K. S. & McMurray, R. G. Calibration of two objective measures of physical activity for children. J. Sports Sci. 26, 1557–1565 (2008).
Freedson, P., Pober, D. & Janz, K. F. Calibration of accelerometer output for children. Med. Sci. Sports Exerc. 37, S523–S530 (2005).
Trost, S. G., Loprinzi, P. D., Moore, R. & Pfeiffer, K. A. Comparison of accelerometer cut points for predicting activity intensity in youth. Med. Sci. Sport. Exerc. 43, 1360–1368 (2011).
Garber, C. E. et al. American College of Sports Medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Med. Sci. Sports Exerc. 43, 1334–1359 (2011).
Meyer, T., Gabriel, H. H. W. & Kindermann, W. Is determination of exercise intensities as percentages of VO2max or HRmax adequate?. Med. Sci. Sport. Exerc. 31, 1342–1345 (1999).
Baldwin, J., Snow, R. J. & Febbraio, M. A. Effect of training status and relative exercise intensity on physiological responses in men. Med. Sci. Sport. Exerc. 32, 1648–1654 (2000).
Armstrong, N. & Barker, A. R. Oxygen uptake kinetics in children and adolescents: A review. Pediatr. Exerc. Sci. 21, 130–147 (2009).
Weatherwax, R. M., Harris, N. K., Kilding, A. E. & Dalleck, L. C. Incidence of VO2max responders to personalized vs standardized exercise prescription. Med. Sci. Sport. Exerc. 51, 681–691 (2019).
Wolpern, A. E., Burgos, D. J., Janot, J. M. & Dalleck, L. C. Is a threshold-based model a superior method to the relative percent concept for establishing individual exercise intensity? A randomized controlled trial. BMC Sports Sci. Med. Rehabil. 7, 1–9 (2015).
Gil-Rey, E., Maldonado-Martín, S., Palacios-Samper, N. & Gorostiaga, E. M. Objectively measured absolute and relative physical activity intensity levels in postmenopausal women. Eur. J. Sport Sci. 19, 539–548 (2019).
Brage, S. et al. Hierarchy of individual calibration levels for heart rate and accelerometry to measure physical activity. J. Appl. Physiol. 103, 682–692 (2007).
Mackintosh, K. A., Fairclough, S. J., Stratton, G. & Ridgers, N. D. A calibration protocol for population-specific accelerometer cut-points in children. PLoS ONE 7, 5–10 (2012).
Boddy, L. M. et al. Individual calibration of accelerometers in children and their health-related implications. J. Sports Sci. 36, 1340–1345 (2018).
Murtagh, E. M., Boreham, C. A. G. & Murphy, M. H. Speed and exercise intensity of recreational walkers. Prev. Med. 35, 397–400 (2002).
Aittasalo, M. et al. Mean amplitude deviation calculated from raw acceleration data: a novel method for classifying the intensity of adolescents’ physical activity irrespective of accelerometer brand. BMC Sports Sci. Med. Rehabil. 7, 18 (2015).
Puyau, M., Adolph, A., Vohra, F. & Butte, N. Validation and calibration of physical activity monitors in children. Obes. Res. 10, 150–157 (2002).
Pate, R. R., Pfeiffer, K. A., Dowda, M., McIver, K. L. & Almeida, M. J. Validation and calibration of an accelerometer in preschool children. Obesity 14, 2000–2006 (2008).
Van Cauwenberghe, E., Labarque, V., Trost, S. G., De Bourdeaudhuij, I. & Cardon, G. Calibration and comparison of accelerometer cut points in preschool children. Int. J. Pediatr. Obes. 6, e582–e589 (2011).
Trost, S. G., Pate, R. R., Pfeiffer, K. A., Sirard, J. R. & Dowda, M. Calibration and evaluation of an objective measure of physical activity in preschool children. J. Phys. Act. Heal. 2, 345–357 (2016).
Radnor, J. M. et al. The influence of growth and maturation on stretch-shortening cycle function in youth. Sport. Med. 48, 57–71 (2018).
Lintu, N. et al. Cardiorespiratory fitness, respiratory function and hemodynamic responses to maximal cycle ergometer exercise test in girls and boys aged 9–11 years: the PANIC Study. Eur. J. Appl. Physiol. 115, 235–243 (2015).
Birat, A. et al. Metabolic and fatigue profiles are comparable between prepubertal children and well-trained adult endurance athletes. Front. Physiol. 9, 387 (2018).
Gao, Y. et al. Sedentary thresholds for accelerometry-based mean amplitude deviation and electromyography amplitude in 7–11 years old children. Front. Physiol. 10, 997 (2019).
Saari, A. et al. New Finnish growth references for children and adolescents aged 0 to 20 years: Length/height-for-age, weight-for-length/height, and body mass index-for-age. Ann. Med. 43, 235–248 (2011).
Ventham, J. C. & Reilly, J. J. Reproducibility of resting metabolic rate measurement in children. Br J Nutr 81, 435–437 (1999).
Saint-Maurice, P. F., Kim, Y., Welk, G. J. & Gaesser, G. A. Kids are not little adults: what MET threshold captures sedentary behavior in children?. Eur. J. Appl. Physiol. 116, 29–38 (2016).
Godfrey, S., Davies, C. T. M., Wozniak, E. & Barnes, C. A. Cardio-respiratory response in normal children. Clin. Sci. 40, 419–431 (1971).
Machado, F. A. & Denadai, B. S. Validity of maximum heart rate prediction equations for children and adolescents. Arq Bras Cardiol 97, 136–140 (2011).
Ruby, B. C. et al. Validity and reliability of combining three methods to determine ventilatory threshold. Med. Sci. Sports Exerc. 33, 1841–1848 (2003).
Laukkanen, A., Pesola, A., Havu, M., Sääkslahti, A. & Finni, T. Relationship between habitual physical activity and gross motor skills is multifaceted in 5- to 8-year-old children. Scand. J. Med. Sci. Sports 24, 102–110 (2013).
Vähä-Ypyä, H., Vasankari, T., Husu, P., Suni, J. & Sievänen, H. A universal, accurate intensity-based classification of different physical activities using raw data of accelerometer. Clin. Physiol. Funct. Imaging 35, 64–70 (2015).
Perkins, N. J. & Schisterman, E. F. The inconsistency of ‘optimal’ cutpoints obtained using two criteria based on the receiver operating characteristic curve. Am. J. Epidemiol. 163, 670–675 (2006).
Acknowledgements
We thank all CHIPASE Study participants for their time and dedication to the study and the Ms Tanja Niemi, MSc and Mr Martti Melin, MSc for helping carrying out the assessments. This study was funded by Ministry of Education and Culture of Finland (OKM/59/626/2016). The authors declare no conflicts of interest.
Author information
Authors and Affiliations
Contributions
E.A.H. analysed the data and drafted the manuscript. Y.G., A.V., and T.R. collected and processed the data for analyses. T.F. planned the research and received funding and contributed to planning the manuscript and interpreting the results and reviewed the manuscript. All authors provided significant intellectual contribution to the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Haapala, E.A., Gao, Y., Vanhala, A. et al. Validity of traditional physical activity intensity calibration methods and the feasibility of self-paced walking and running on individualised calibration of physical activity intensity in children. Sci Rep 10, 11031 (2020). https://doi.org/10.1038/s41598-020-67983-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-67983-7
This article is cited by
-
Associations of fitness, motor competence, and adiposity with the indicators of physical activity intensity during different physical activities in children
Scientific Reports (2021)
-
Physical activity accumulation along the intensity spectrum differs between children and adults
European Journal of Applied Physiology (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.