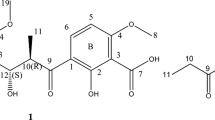
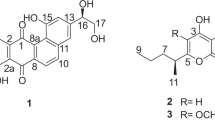
ABSTRACT
Polycyclic xanthones are secondary metabolites from actinomycetes and cervinomycin A and B are bioactive 26-membered polycyclic xanthones from Streptomyces sp. CPCC 204980. Herein, we report cervinomycins C1-4 (1–4) from the same strain. The structures of 1–4 were determined by 1D- and 2D-NMR, or single-crystal X-ray diffraction. Compounds 1–4 feature the open or loss of A (oxazolidine) ring in their angular polycyclic framework compared with cervinomycin B. Compounds 1–4 showed potent cytotoxicity against human cancer cell lines HCT116 and BxPC-3, with IC50 at 0.9–801.0 nM and strong anti-Gram-positive bacterial activity.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Peres V, Nagem TJ, de Oliveira FF. Tetraoxygenated naturally occurring xanthones. Phytochemistry 2000;55:683–710.
Masters KS, Bräse S. Xanthones from fungi, lichens, and bacteria: the natural products and their synthesis. Chem Rev. 2012;112:3717–76.
Winter DK, Sloman DL, Porco JA Jr. Polycyclic xanthone natural products: structure, biological activity and chemical synthesis. Nat Prod Rep. 2013;30:382–91.
Peoples AJ, et al. Neocitreamicins I and II, novel antibiotics with activity against methicillin resistant Staphylococcus aureus and vancomycin-resistant Enterococci. J Antibiot (Tokyo). 2008;61:457–63.
Kang HS, Brady SF, Arixanthomycins A-C. Phylogeny-guided discovery of biologically active eDNA-derived pentangular polyphenols. ACS Chem Biol. 2014;9:1267–72.
Ratnayake R. Kibdelones: novel anticancer polyketides from a rare Australian actinomycete. Chemistry 2007;13:1610–1609.
Gurevich AI, et al. The structure of albofungin. Tetrahedron Lett. 1972;13:1751–4.
Fukushima K, Ishiwata K, Kuroda S, Arai T. Identity of antibiotic P-42-1 elaborated by Actinomyces tumemacerans with kanchanomycin and albofungin. J Antibiot (Tokyo). 1973;26:65–69.
Drautz H, Keller-Schierlein W, Zahner H. Metabolic products of microorganisms, 149. Lysolipin I, a new antibiotic from Streptomyces violaceoniger (author’s transl). Arch Microbiol. 1975;106:175–90.
Lopez P, et al. Isolation of the lysolipin gene cluster of Streptomyces tendae Tü 4042. Gene 2010;461:5–14.
Omura S, et al. Cervinomycin A1 and A2, new antibiotics active against anaerobes, produced by Streptomyces cervinus sp. nov. J Antibiot (Tokyo). 1982;35:645–52.
Nakagawa A, Iwai Y, Shimizu H, Omura S. Enhanced antimicrobial activity of acetyl derivatives of cervinomycin. J Antibiot (Tokyo). 1986;39:1636–8.
Nakagawa A, et al. Structure of cervinomycin, a novel xantone antibiotic active against anaerobe and mycoplasma. J Antibiot (Tokyo). 1987;40:301–8.
Carter GT, Nietsche JA, Williams DR, Borders DB. Citreamicins, novel antibiotics from Micromonospora citrea: isolation, characterization, and structure determination. J Antibiot (Tokyo). 1990;43:504–12.
Wu S, et al. Xantholipin B produced by the stnR inactivation mutant Streptomyces flocculus CGMCC 4.1223 WJN-1. J Antibiot (Tokyo). 2017;70:90–5.
Zhang W, et al. Unveiling the post-PKS redox tailoring steps in biosynthesis of the type II polyketide antitumor antibiotic xantholipin. Chem Biol 2012;19:422–32.
Rujirawanich J, et al. Synthesis and biological evaluation of kibdelone C and its simplified derivatives. J Am Chem Soc. 2016;138:10561–70.
Kong L, et al. A multifunctional monooxygenase XanO4 catalyzes xanthone formation in xantholipin biosynthesis via a cryptic demethoxylation. Cell Chem Biol. 2016;23:508–16.
Kudo F. Cloning of the biosynthetic gene cluster for naphthoxanthene antibiotic FD-594 from Streptomyces sp. TA-0256. J Antibiot (Tokyo). 2011;64:123–32.
Hu X, et al. Cytotoxic and antibacterial cervinomycins B1-4 from a Streptomyces species. J Nat Prod. 2019;82:2337–42.
Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing. Twenty-seventh informational supplement. M100-S27. Wayne, PA: Clinical and Laboratory Standards Institute (CLSI); 2017.
Clinical and Laboratory Standards Institute. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. M07-A10. Wayne, PA: Clinical and Laboratory Standards Institute (CLSI); 2015.
Acknowledgements
We thank colleagues at the Nuclear Magnetic Resonance Center (Institute of Materia Medica, CAMS & PUMC) and Analytical Center of Drugs (Institute of Medicinal Biotechnology, CAMS & PUMC) for the NMR, MS, and CD analyses. This work was supported by CAMS Initiative for Innovative Medicine (CAMS-I2M-1-012), National Natural Science Foundation of China (81573328), Drug Innovation Major Project (2018ZX09711-001), and National Infrastructure of Microbiological Resources (No. NIMR-2018-3).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Hu, X., Sun, W., Li, S. et al. Cervinomycins C1-4 with cytotoxic and antibacterial activity from Streptomyces sp. CPCC 204980. J Antibiot 73, 812–817 (2020). https://doi.org/10.1038/s41429-020-0342-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41429-020-0342-1