Rapid Sampling of Suspended and Floating Microplastics in Challenging Riverine and Coastal Water Environments in Japan
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sampling Process
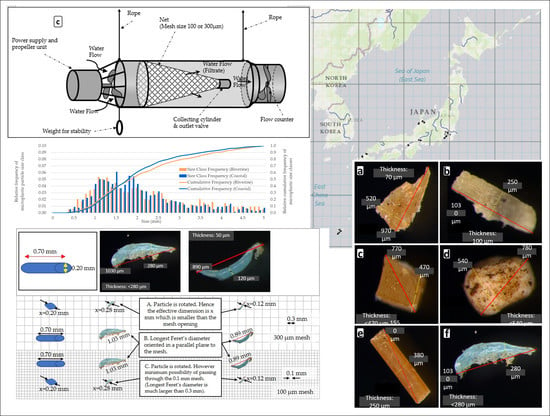
2.1.1. Sampling Devices
2.1.2. Sampling Locations
2.2. Analytical Process
2.2.1. Sample Extraction and Purification
2.2.2. Contamination Control
2.2.3. Size Measurement and Polymer Confirmation
2.3. Calculations
2.3.1. Calculation of the Flow-Volume through the Devices
- V:
- volume passed through the net,
- D:
- net mouth or tube diameter (for BS-PN: D = 24 cm, AM-5: D = 22 cm and for AM-6: D = 18 cm),
- a:
- flow counter reading,
- b:
- conversion coefficient (manufacturers value for the corresponding impellers; in this case for the impellers used for BS-PN, AM-5, and AM-6: b = 5.12),
- π
- = 3.14.
2.3.2. Microplastic Particle Size Distribution
- fx:
- relative frequency of microplastic particles in size class x
- nx:
- number of microplastic particles in size class x
- N:
- total number of microplastic particles
2.4. Statistical Analyses
- t:
- the ratio of the departure of the estimated value of mean particle size from its hypothesized value to its standard error
- Mr:
- mean particle size of riverine microplastic
- Mc:
- mean particle size of coastal microplastic
- sr:
- standard deviation for riverine microplastics
- sc:
- standard deviation for coastal microplastics
- Nr:
- total number of riverine microplastic particles
- Nc:
- total number of coastal microplastic particles
3. Results
3.1. Operational Conditions of the Sampling Devices
3.2. Microplastic Concentrations Measured with Different Sampling Devices
3.3. Microplastic Particle Size Distributions for the Sampling in Japanese Riverine and Coastal Environments Using AM-6(300)
3.4. Plastic Polymer Compositions in River and Coastal Areas of Japan
4. Discussion
4.1. Operational Conditions of the Sampling Devices
4.2. Microplastic Concentrations Measured with Different Sampling Devices
4.3. Relative Particle Size Distribution of Microplastic Pieces Sampled Using AM-6 in Japanese Riverine and Coastal Environments
4.4. Plastic Compositions in River and Coastal Areas of Japan
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Thompson, R.C.; Barnes, D.K.A.; Galgani, F.; Barlaz, M. Accumulation and fragmentation of plastic debris in global environments. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 1985–1998. [Google Scholar]
- Horton, A.A.; Jürgens, M.D.; Lahive, E.; van Bodegom, P.M.; Vijver, M.G. The influence of exposure and physiology on microplastic ingestion by the freshwater fish Rutilus rutilus (roach) in the River Thames, UK. Environ. Pollut. 2018, 236, 188–194. [Google Scholar] [CrossRef] [PubMed]
- Bellasi, A.; Binda, G.; Pozzi, A.; Galafassi, S.; Volta, P.; Bettinetti, R. Microplastic contamination in freshwater environments: A review, focusing on interactions with sediments and benthic organisms. Environments 2020, 7, 30. [Google Scholar] [CrossRef] [Green Version]
- Jemec, A.; Horvat, P.; Kunej, U.; Bele, M.; Kržan, A. Uptake and effects of microplastic textile fibers on freshwater crustacean Daphnia magna. Environ. Pollut. 2016, 219, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Redondo-Hasselerharm, P.E.; Falahudin, D.; Peeters, E.T.; Koelmans, A.A. Microplastic effect thresholds for freshwater benthic macroinvertebrates. Environ. Sci. Technol. 2018, 52, 2278–2286. [Google Scholar] [CrossRef] [PubMed]
- Su, L.; Cai, H.; Kolandhasamy, P.; Wu, C.; Rochman, C.M.; Shi, H. Using the Asian clam as an indicator of microplastic pollution in freshwater ecosystems. Environ. Pollut. 2018, 234, 347–355. [Google Scholar] [CrossRef] [PubMed]
- Lebreton, L.C.M.; van der Zwet, J.; Damsteeg, J.-W.; Slat, B.; Andrady, A.; Reisser, J. River plastic emissions to the world’s oceans. Nat. Commun. 2017, 8, 15611. [Google Scholar] [CrossRef]
- Schmidt, C.; Krauth, T.; Wagner, S. Export of plastic debris by rivers into the sea. Environ. Sci. Technol. 2017, 51, 12246–12253. [Google Scholar] [CrossRef]
- Ryberg, M.; Laurent, A.; Hauschild, M.Z. Mapping of Global Plastic Value Chain and Plastic Losses to the Environment: With a Particular Focus on Marine Environment; United Nations Environment Programme: Nairobi, Kenya, 2018. [Google Scholar]
- Isobe, A.; Iwasaki, S.; Uchida, K.; Tokai, T. Abundance of non-conservative microplastics in the upper ocean from 1957 to 2066. Nat. Commun. 2019, 10, 417. [Google Scholar] [CrossRef] [Green Version]
- Cozzolino, L.; Nicastro, K.R.; Zardi, G.I.; Carmen, B. Species-specific plastic accumulation in the sediment and canopy of coastal vegetated habitats. Sci. Total Environ. 2020, 723, 138018. [Google Scholar] [CrossRef]
- Microplastics. Encyclopædia Britannica. Available online: www.britannica.com/technology/microplastic (accessed on 8 May 2020).
- Hakkarainen, M.; Albertsson, A.C. Environmental Degradation of Polyethylene. In Long Term Properties of Polyolefins, 1st ed.; Albertsson, A.C., Ed.; Springer: Berlin/Heidelberg, Germany; New York, NY, USA, 2004; Volume 169, pp. 177–200. [Google Scholar]
- Van Emmerik, T.; Schwarz, A. Plastic debris in rivers. Wires Water 2020, 7, 1398. [Google Scholar] [CrossRef] [Green Version]
- Anderson, J.C.; Park, B.J.; Palace, V.P. Microplastics in aquatic environments: Implications for Canadian ecosystems. Environ. Pollut. 2016, 218, 269–280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kowalski, N.; Reichardt, A.M.; Waniek, J.J. Sinking rates of microplastics and potential implications of their alteration by physical, biological, and chemical factors. Mar. Pollut. Bull. 2016, 109, 310–319. [Google Scholar] [CrossRef] [PubMed]
- Koelmans, A.A.; Nor, N.H.M.; Hermsen, E.; Kooi, M.; Mintenig, S.M.; De France, J. Microplastics in freshwaters and drinking water: Critical review and assessment of data quality. Water Res. 2019, 155, 410–422. [Google Scholar] [CrossRef] [PubMed]
- Van Emmerik, T.; Kieu-Le, T.C.; Loozen, M.; Oeveren, K.; Strady, E.; Bui, X.T.; Egger, M.; Gasperi, J.; Lebreton, L.; Nguyen, P.D.; et al. A methodology to characterize riverine macroplastic emission into the ocean. Front. Mar. Sci. 2018, 5, 372. [Google Scholar] [CrossRef]
- Scircle, A.; Cizdziel, J.V.; Missling, K.; Li, L.; Vianello, A. Single-Pot Method for the Collection and Preparation of Natural Water for Microplastic Analyses: Microplastics in the Mississippi River System during and after Historic Flooding. Environ. Toxicol. Chem. 2020, 39, 986–995. [Google Scholar] [CrossRef]
- Alam, F.C.; Sembiring, E.; Muntalif, B.S.; Suendo, V. Microplastic distribution in surface water and sediment river around slum and industrial area (case study: Ciwalengke River, Majalaya district, Indonesia. Chemosphere 2019, 224, 637–645. [Google Scholar] [CrossRef]
- Lima, A.R.A.; Barletta, M.; Costa, M.F. Seasonal distribution and interactions between plankton and microplastics in a tropical estuary. Estuar. Coast. Shelf Sci. 2015, 165, 213–225. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, J.; Xie, Y.; Zhong, S.; Yang, B.; Lu, D.; Zhong, Q. Distribution of microplastics in surface water and sediments of Qin river in Beibu Gulf, China. Sci. Total Environ. 2020, 708, 135176. [Google Scholar] [CrossRef]
- Maes, T.; van der Meulen, M.D.; Devriese, L.I.; Leslie, H.A.; Huvet, A.; Frère, L.; Vethaak, A.D. Microplastics baseline surveys at the water surface and in sediments of the North-East Atlantic. Front. Mar. Sci. 2017, 4, 135. [Google Scholar] [CrossRef] [Green Version]
- Bordós, G.; Urbányi, B.; Micsinai, A.; Kriszt, B.; Palotai, Z.; Szabó, I.; Hantosi, Z.; Szoboszlay, S. Identification of microplastics in fishponds and natural freshwater environments of the Carpathian basin, Europe. Chemosphere 2019, 216, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Kataoka, T.; Nihei, Y.; Kudou, K.; Hinata, H. Assessment of the sources and inflow processes of microplastics in the river environments of Japan. Environ. Pollut. 2019, 244, 958–965. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Lu, Z.; Zheng, H.; Wang, J.; Chen, C. Microplastics in surface water and sediments of Chongming Island in the Yangtze Estuary, China. Environ. Sci. Eur. 2020, 32, 15. [Google Scholar] [CrossRef] [Green Version]
- Rochman, C.M.; Brookson, C.; Bikker, J.; Djuric, N.; Earn, A.; Bucci, K.; Athey, S.; Huntington, A.; McIlwraith, H.; Munno, K.; et al. Rethinking microplastics as a diverse contaminant suite. Environ. Toxicol. Chem. 2019, 38, 703–711. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hidalgo-Ruz, V.; Gutow, L.; Thompson, R.C.; Thiel, M. Microplastics in the marine environment: A review of the methods used for identification and quantification. Environ. Sci. Technol. 2012, 46, 3060–3075. [Google Scholar] [CrossRef] [PubMed]
- Rose, D.; Webber, M. Characterization of microplastics in the surface waters of Kingston Harbour. Sci. Total Environ. 2019, 664, 753–760. [Google Scholar] [CrossRef] [PubMed]
- Isobe, A.; Uchida, K.; Tokai, T.; Iwasaki, S. East Asian seas: A hot spot of pelagic microplastics. Mar. Pollut. Bull. 2015, 101, 618–623. [Google Scholar] [CrossRef] [PubMed]
- Kapp, K.J.; Yeatman, E. Microplastic hotspots in the Snake and Lower Columbia rivers: A journey from the Greater Yellowstone Ecosystem to the Pacific Ocean. Environ. Pollut. 2018, 241, 1082–1090. [Google Scholar] [CrossRef]
- Briggs, E.; de Moura, E.A.B.; Furusawa, H.A.; Cotrim, M.E.B.; Oguzie, E.E.; Lugao, A.B. Microplastics: A novel method for surface water sampling and sample extraction in Elechi Creek, Rivers State, Nigeria. In Characterization of Minerals, Metals, and Materials; Springer International Publishing: Cham, Switzerland, 2019; pp. 269–281. [Google Scholar]
- Naidoo, T.; Glassom, D.; Smit, A.J. Plastic pollution in five urban estuaries of KwaZulu-Natal South Africa. Mar. Pollut. Bull. 2015, 101, 473–480. [Google Scholar] [CrossRef]
- McEachern, K.; Alegria, H.; Kalagher, A.L.; Hansen, C.; Morrison, S.; Hastings, D. Microplastics in Tampa Bay, Florida: Abundance and variability in estuarine waters and sediments. Mar. Pollut. Bull. 2019, 148, 97–106. [Google Scholar] [CrossRef]
- Besseling, E.; Redondo-Hasselerharm, P.; Foekema, E.M.; Koelmans, A.A. Quantifying ecological risks of aquatic micro-and nanoplastic. Crit. Rev. Environ. Sci. Technol. 2019, 49, 32–80. [Google Scholar] [CrossRef] [Green Version]
- Jung, M.R.; Horgen, F.D.; Orski, S.V.; Rodriguez, V.; Beers, K.L.; Balazs, G.H.; Jones, T.T.; Work, T.M.; Brignac, K.C.; Royer, S.J.; et al. Validation of ATR FT-IR to identify polymers of plastic marine debris, including those ingested by marine organisms. Mar. Pollut. Bull. 2018, 127, 704–716. [Google Scholar] [CrossRef] [PubMed]
- Enders, K.; Lenz, R.; Stedmon, C.A.; Nielsen, T.G. Abundance, size and polymer composition of marine microplastics ≥10 μm in the Atlantic Ocean and their modelled vertical distribution. Mar. Pollut. Bull. 2015, 100, 70–81. [Google Scholar] [CrossRef] [PubMed]
- Isobe, A.; Uchiyama-Matsumoto, K.; Uchida, K.; Tokai, T. Microplastics in the Southern Ocean. Mar. Pollut. Bull. 2017, 114, 623–626. [Google Scholar] [CrossRef]
- Lambert, S.; Wagner, M. Microplastics are contaminants of emerging concern in freshwater environments: An overview. In Freshwater Microplastics: Emerging Environmental Contaminants; Wagner, M., Lambert, S., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 1–23. [Google Scholar]
- Sighicelli, M.; Pietrelli, L.; Lecce, F.; Iannilli, V.; Falconieri, M.; Coscia, L.; Di Vito, S.; Nuglio, S.; Zampetti, G. Microplastic pollution in the surface waters of Italian Subalpine Lakes. Environ. Pollut. 2018, 236, 645–651. [Google Scholar] [CrossRef]
- Campanale, C.; Stock, F.; Massarelli, C.; Kochleus, C.; Bagnuolo, G.; Reifferscheid, G.; Uricchio, V.F. Microplastics and their possible sources: The example of Ofanto river in Southeast Italy. Environ. Pollut. 2020, 258, 113284. [Google Scholar] [CrossRef]
- Dris, R.; Gasperi, J.; Rocher, V.; Tassin, B. Synthetic and non-synthetic anthropogenic fibers in a river under the impact of Paris Megacity: Sampling methodological aspects and flux estimations. Sci. Total Environ. 2018, 618, 157–164. [Google Scholar] [CrossRef] [Green Version]







| Scenario | BS-PN | AM-5 | AM-6 | ||||
|---|---|---|---|---|---|---|---|
| Parameter | 100 µm | 300 µm | 100 µm | 300 µm | 100 µm | 300 µm | |
| Operated flowrate range (m3/min) | 0.11 to 0.67 a | 0.17 to 1.00 a | 2.5 to 7.0 b | 2.5 to 7.0 b | 2.5 to 7.0 b | 2.5 to 7.0 b | |
| Sampling duration (min) | 15–90 | 10–60 | 3 | 3 | 3 | 3 | |
| Minimum human resources required (# people) | 1–2 | 1–2 | 1–2 | 1–2 | 2–3 | 2–3 | |
| Effect of net clogging on flow volume measurement | The difference between river flowrate and filtration rate increases. Partial tilt-up the net. Volume measurement errors. | Flow counter at the center moves along with the forced inflow. Volume measurement errors. | Flow counter is located at the filtrate side. It measures only the filtered volume. Minimum volume measurement errors. | ||||
| Device and Method Facts | Sampling Environment | Concentration (Particles/m3) | Reference |
|---|---|---|---|
| Trawling a 300 µm net. (Sampling volume = 100 m3). | Estuary, Brazil | 0.1 | [21] |
| Trawling 333 µm net. | River, China | 0.1–5.6 | [22] |
| Trawling a 333 µm net. | Channel, UK | 0–1.5 | [23] |
| Jet pump and external power supply unit (sampling volume ≈ 1.5 m3). Complex system due to the requirement of the crew to carry equipment, boat travel, pumping out, and filtration through the sieves. | Riverine, Hungary | 3.52–32.05 | [24] |
| Bridge suspended net (300 µm); 5 to 30 min sampling durations. Capable only with rivers with high flow velocity and overhead bridges. | Riverine, Japan | 1.6 ± 2.3 | [25] |
| Vertically immersed filter with suction pump form the bottom 300 µm; 8 to 13 min taken for volume 0.1 m3 sampling. | Estuary, China | 67.5 | [26] |
| Trawling a 300 µm net. | Riverine, Nigeria | 0–0.2 | [32] |
| 100 µm net kept across the river flow (1–2 min duration). (V = 3.2 m3). Longer sampling led to issues of clogging with organic matter. Capable only with rivers with high flow velocity. | Riverine, Wayni, USA | 0–13.7 | [31] |
| Trawling a 300 µm net Sampling volume = 10 m3. | Estuary, South Africa | 1–7 | [33] |
| Trawling a 330 µm net. | Bay, USA | 4.5 ± 2.3 | [34] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abeynayaka, A.; Kojima, F.; Miwa, Y.; Ito, N.; Nihei, Y.; Fukunaga, Y.; Yashima, Y.; Itsubo, N. Rapid Sampling of Suspended and Floating Microplastics in Challenging Riverine and Coastal Water Environments in Japan. Water 2020, 12, 1903. https://doi.org/10.3390/w12071903
Abeynayaka A, Kojima F, Miwa Y, Ito N, Nihei Y, Fukunaga Y, Yashima Y, Itsubo N. Rapid Sampling of Suspended and Floating Microplastics in Challenging Riverine and Coastal Water Environments in Japan. Water. 2020; 12(7):1903. https://doi.org/10.3390/w12071903
Chicago/Turabian StyleAbeynayaka, Amila, Fujio Kojima, Yoshikazu Miwa, Nobuhiro Ito, Yasuo Nihei, Yu Fukunaga, Yuga Yashima, and Norihiro Itsubo. 2020. "Rapid Sampling of Suspended and Floating Microplastics in Challenging Riverine and Coastal Water Environments in Japan" Water 12, no. 7: 1903. https://doi.org/10.3390/w12071903