Abstract
The study describes the development and employment of plant tests based on artificial inoculation of seeds or the potting substrate for evaluating the potential of microorganisms to control seedling blight of maize caused by seed- and soil-borne fusaria. Nine strains of Fusarium were isolated from maize kernels and identified morphologically and by molecular methods as belonging to the species Fusarium verticillioides, F. subglutinans, F. cerealis, F. poae and F. proliferatum. In order to determine pathogenicity, maize kernels were inoculated by immersion in suspensions of conidia of these isolates and sown in a pasteurized substrate in seed trays. Based on plant dry weight, the isolates of F. verticillioides and F. subglutinans were more pathogenic than the other isolates. Using an isolate of F. subglutinans, the efficacy of a set of 25 potential fungal and bacterial antagonists was assessed using inoculation of maize kernels by placement in mixtures of the pathogen and the antagonists. The results obtained with this methodology indicate the potential of a number of different microorganisms applied as seed treatments, including some reported previously as biocontrol agents, to control seed-borne seedling blight of maize. In order to develop a method for the testing of biocontrol agents against soil-borne attack, isolates of F. subglutinans, F. cerealis and F poae from maize kernels together with isolates of F. avenaceum, F. culmorum and F. graminearum originating from maize silage and wheat were used to artificially inoculate the potting substrate. The results showed large differences in pathogenicity, with the most aggressive isolates belonging to F. culmorum and F. graminearum.
Similar content being viewed by others
Introduction
In order to avoid damage during germination and early crop establishment by insect pests and plant pathogens, seeds of many agricultural and horticultural crops are routinely treated before they are sown. The most common method is seed dressing with chemical compounds having insecticidal or fungicidal activity.
Primarily driven by concern about adverse effects of chemical plant protection products on humans and the environment, efforts have been made in recent decades to replace chemical seed treatments by environmentally more friendly methods. The alternatives available so far include physical methods, the use of living bacteria or fungi and natural compounds from plants and microorganisms (Mancini and Romanazzi 2014; Koch and Roberts 2014; O`Callaghan 2016). With the continuing call by the public, consumers and authorities for a reduction in chemical pesticide use on the one hand, and discontinuation of authorisations for chemical active ingredients on the other, along with a lack of new registrations, it can be expected that non-chemical seed treatments will gain importance in the future. However, non-chemical seed treatments are still not available in many economically important crops, necessitating further research in this field.
In maize, fungicidal seed treatments are primarily applied to control pathogenic fungi of the genus Fusarium, which can be both seed- and soil-borne (Munkvold and White 2016). Generally, the relative importance of soil-borne versus seed-borne infections by fusaria in maize is unknown and may vary depending on site, weather conditions and seed quality. It is therefore imperative that fungicidal seed treatments control both routes of infection. Screening and development of new seed treatment agents should preferably be performed under conditions resembling the practical conditions of use. Consequently, the activity against seed-borne pathogens should ideally be assessed using naturally infested seeds. However, if infested seed lots are not available or vary in characters that affect the outcome of the experiment, such as degree of infection and composition and localization of the pathogens, the seeds can be artificially inoculated. Depending on the pathosystem, the inoculum generally consists of dry spores (like in Tilletia caries) or, more commonly, spore suspensions (e.g., suspensions of conidia). Artificial inoculation is frequently used for seed treatment trials with certain bunts and smuts of small grain cereals (Nielsen 1976; Nagy and Moldovan 2007). It has also been employed with other pathogenic fungi, including fusaria, for evaluating the effect of seed treatments (Galperin et al. 2003; Bressan and Figueiredo 2005) as well as in the context of epidemiology (Moussart et al. 1998), characterization of pathogenicity (Imathiu et al. 2010) and identification of resistant germ plasm (Bacon et al. 1994; Browne and Cooke 2005; Maitlo et al. 2016). In most studies, seed inoculation with fusaria has been performed by immersion of seeds in conidial suspensions or by spraying the conidial suspensions over the seeds (Bacon and Hinton 1996). Before inoculation, the seed surface should be disinfected or the seeds subjected to a heat treatment in order to remove internal seed-borne microorganisms (Bacon et al. 1994; Galperin et al. 2003).
Efficacy testing of seed treatments against soil-borne fusaria is generally done using artificial inoculation of the soil or potting substrate. Commonly, the pathogen inoculum consists of pre-colonized substrates such as intact or milled cereals or mixtures of the latter with sand that are evenly mixed with the soil or potting substrate (Mao et al. 1998) or applied as a layer on the top (Pandey et al. 2001; Munkvold and O`Mara 2002). Alternatively, conidial suspensions of the pathogen have been applied to the potting medium (Bacon and Hinton 2007). The advantage of artificial inoculation compared to the use of field soil is the opportunity to employ known pathogen strains and to vary the infection pressure by adjusting the inoculum concentration (Mao et al. 1998; Maitlo et al. 2016). This, however, requires pre-tests defining the test conditions, especially with regard to the pathogenicity of the Fusarium strains in question.
The aim of the present work was to establish model tests for evaluating bacteria and fungi as seed treatments against seed- and soil-borne fusaria on maize. For this purpose, the ability of different fusaria to cause seedling blight was determined under controlled conditions in tests with artificial inoculation of the seeds or artificial inoculation of the potting substrate, respectively. The test conditions were optimized, and the efficacy of a set of 25 bacterial and fungal strains against seed-borne fusaria was evaluated by co-inoculating maize kernels with an isolate of F. subglutinans and the putative control agents. Under the conditions employed, treatment with the majority of strains improved seedling development compared to the controls (= seed inoculation with the pathogen only), indicating that they may have potential for use as seed treatments.
Materials and methods
Fusaria
The isolates of Fusarium employed were obtained from the culture collection of Kiel University originating from maize silage (F. graminearum Ck3, F. graminearum Os12, F. culmorum Fu13, F. avenaceum To8) and wheat kernels (F. culmorum VIII18). In addition, new isolates were freshly isolated from maize kernels. For this purpose, maize kernels were surface disinfected by immersion for 10 min in NaOCl (1%), washed in sterile distilled water, placed on moist filter paper and incubated at room temperature for 3–7 days. Mycelia growing from the kernels were removed with a needle and streaked on PDA. Pure cultures were established from isolates suspected to be fusaria and stored in a freezer at 80 °C until use.
Species determination was carried out based on morphology after culturing on SNA (Leslie and Summerell 2006) and molecularly by using universal and species-specific marker genes. DNA was extracted with the DNeasy Plant Mini Kit(Quiagen) for PCR amplification of the universal marker genes for elongation factor 1 alpha (TEF-1α) and ITS-5f and ITS-4r (White et al. 1990). Mycelium was carefully removed from PDA cultures and homogenized with a FastPrep-24®Tissue and Cell Homogenizer (MP Biomedicals, USA) using Lysing Matrix A and a run time of 20 s with an intensity of 4 m/sec. Thereafter, the extraction kit protocol was followed. The amount and purity of the gDNA samples were determined using the NanoDrop 2000c (Thermosientific, USA), reaching an average of about 100 ng/µl per sample. For the PCR reactions, the Phusion HighFidelety Polymerase (NewEnglandBiolabs, USA) was used with an annealing temperature of 68 °C and 40 cycles. Microsynth SEQLAB (Germany) performed the sequencing according to Sanger. The sequences were analyzed by comparison with similar sequences in the NCBI database.
For molecular species determination with species-specific primers, DNA was extracted using the CTAB method. Mycelial tissue, 50 µg, was ground in a mill and incubated in CTAB buffer for 1 h at 65 °C. RNAse A, 2 µl, was added and samples were incubated at 37 °C for 30 min. Five hundred microliters of chloroform-isoamylalcohol (24 + 1) were added and samples were shaken vigorously for 10 min and centrifuged at 14,000 rpm for 10 min. The upper phase was carefully transferred to a new tube and extracted with 700 µl isopropanol. Samples were incubated on ice for 30 min, centrifuged and the pellet was cleaned twice with a) EtOH 76%, NA-acetate 0.2 M and b) EtOH 76%, Na- acetate 10 mM. The pellet was dried and dissolved in water. The following species-specific primers were used: JIA-F and JIA-R (F. avenaceum; Turner et al. 1998), Fc01-F and Fc01-R (F. culmorum; Nicholson et al. 1998), Fg16-F and Fg16-R (F. graminearum; Nicholson et al. 1998), CLOX-F and CLOX-R (F. oxysporum; Mulè et al. 2004b), Fp82-f and Fp82R (F. poae; Parry and Nicholson 1996), PRO-1-F and PRO-2-R (F. proliferatum; Mulè et al. 2004a), SUB1 and SUB2 (F. subglutinans; Mulè et al. 2004a), and VERT-1-F and VERT-2-R (F. verticillioides; Patiño et al. 2004).
Potential antagonists
The microorganisms tested for activity against seed-inoculated F. subglutinans were taken from the culture collection of the Julius Kühn-Institut (Institute for Biological Control) or supplied by co-operating scientists. About one-third of the tested microorganisms had a previous record as antagonists of plant pathogens (Table 1).
Preparation of Fusarium inoculum and application to seeds
For the preparation of pathogen inoculum, the fusaria were cultured on potato dextrose agar (PDA; Sigma-Aldrich, Taufkirchen, Germany). Plates with sporulating cultures were flooded with 10 ml sterile distilled water (0.0125% Tween® 80; Sigma-Aldrich, Taufkirchen, Germany) and conidia were dislodged by gently scraping the colony surface with a spatula. The suspensions were filtered through cotton gauze (Mullro®) to remove mycelial fragments, and spore concentrations were determined with a hemocytometer. Depending on the amount of spores present, the concentration of the resulting suspensions was adjusted to 1 × 105 (F. cerealis), 1 × 106 (F. poae) or 1 × 107 (all other fusaria) conidia per ml. Methyl cellulose (1%) was routinely added to all conidial or bacterial suspensions used for seed inoculation.
In the experiment investigating the effect of the spore load, the concentration of microconidia of F. verticilliodes (I) in the inoculum suspension was adjusted to 1 × 105, 1 × 106, 1 × 107 or 1 × 108 per ml. Maize kernels (Zea mays cv. “Padrino”) were disinfected as described above and inoculated by placement of 100 kernels each for 10 min in 50 ml of the conidial suspensions of the pathogens. The kernels were then dried overnight in a laminar flow hood and sown the next day, except in one experiment where only half of the inoculated kernels were sown the following day and the other half were kept in a paper bag for 80 days in a refrigerator before they were sown.
Preparation of antagonist inoculum and application to seeds
Putative microbial antagonists were applied in mixtures with conidial suspensions of the pathogen F. subglutinans (I). Inocula of the antagonists were prepared from cells or spores freshly raised on Petri plates or in shake cultures, except for P. chlororaphis MA342 which was applied using the commercial formulation Cerall®. Streptomyces antimycoticus FZB 53 was grown for 3 weeks in Petri plates on potato medium (Koch and Löffler 2009), all other bacterial antagonists were cultured for 48 h on a rotary shaker at 25 °C in Tryptic Soy Broth (Carl Roth, Karlsruhe, Germany). Aliquots of the shake cultures or the product Cerall® were added to conidial suspensions of F. subglutinans (I) (concentration 1 × 108/microconidia per ml; prepared as described above) in the ratio of 1:10, resulting in a final concentration of 1 × 107 microconidia per ml. The putative fungal antagonists were grown in Petri plates (9 cm diameter) on PDA. To sporulating fungal cultures and sporulating plates of the actinomycete S. antimycoticus FZB 53, 10 ml sterile distilled water (0,0125% Tween 80®) were added. Spore suspensions were then prepared, and 1:10 dilutions with the Fusarium inoculum made, as described above. The resulting final spore concentration in the inoculum suspension of the fungal antagonists was 1–3 × 107 conidia per ml, except for F. solani where it was 3 × 106 conidia per ml and P. moreaui (1 × 106 ascospores per ml). Disinfected maize kernels (Zea mays cv. “Padrino”) were placed for 10 min in the antagonist-pathogen mixtures described, dried overnight under a laminar flow and sown the following day. The pathogen controls were seeds placed in suspensions containing only conidia of F. subglutinans (I) (1 × 107 conidia per ml). The chemical seed treatment fungicide Maxim XL (25 g/l fludioxonil, 10 g/l metalaxyl-M) was used according to the recommendations of the manufacturer. It was applied on inoculated, dried seeds of the pathogen controls one day after inoculation.
Preparation of Fusarium inoculum for soil application
Fusarium isolates were grown on millet seeds, buckwheat, or pearl barley (all food grade). Conidial suspensions were prepared as described above, adjusted to 1 × 105 per ml and diluted with sterile water 1:6. The suspensions obtained were added to 100 g autoclaved millet seeds, buckwheat or pearl barley, respectively, in 1-l Erlenmeyer flasks at a rate of 30 ml per flask. The flasks were incubated in darkness at 20 °C for 72 h and agitated once a day to prevent the formation of clots.
Growth room trials
Horticultural substrate (Fruhstorfer Erde Typ P; Hawita Gruppe GmbH, Vechta, Germany) was mixed with sand (60:40, [w/w]), incubated for 48 h at 60 °C, and after cooling adjusted to a gravimetric water content of 0.45. In the experiments with inoculated seeds, the potting substrate was filled in 27.5 × 17.5 × 7 cm household polypropylene containers (“seed trays”) with 2–4 seed trays per treatment. Twenty-five maize seeds were sown per seed tray and inoculated with fusaria or a mixture of fusaria and microbial antagonists as described above.
Potting substrate inoculated with fusaria was prepared by carefully mixing the colonized millet, buckwheat or pearl barley-inoculum described above into the potting substrate at concentrations of 1 or 3% (w/w). The experiments with inoculated potting substrate were performed in 10 × 10 cm plastic pots. Per treatment, 3–5 pots with 6 maize seeds (cv. “Emmy”) each were employed.
After sowing, the seed trays/pots were covered with a layer (approx. 1 cm thick) of vermiculite and their weight was recorded before they were placed in a plant growth room under 16 h of light from fluorescent lamps at 20 °C. During the course of the experiment, the seed trays/pots were watered according to weight. Two weeks after planting the number of plants per seed tray/pot was recorded, plants were cut at the crown, and their dry weight was recorded after incubation for 48 h at 80 °C. The results are presented as number of plants or plant dry weight per seed tray or pot, respectively.
Statistical analysis
In all growth room experiments pots were arranged in a randomized design. Data were analyzed applying one-way ANOVA using the statistical software “R”. Homogeneity of variances was asserted using Levene’s Test (p ≤ 0.05). The Tukey test was used for comparisons between treatments. A p ≤ 0.05 significance level was used throughout.
Results
PCR sequencing, species-specific primers and morphological characters were used to identify fusaria from surface-disinfected maize kernels. Of the eight isolates studied, two were identified as F. verticillioides (F. verticillioides [I], F. verticillioides [II]), three as F. subglutinans (F. subglutinans [I], F. subglutinans [II], F. subglutinans [III]), and one each as F. cerealis, F. proliferatum and F. poae, respectively.
The pathogenicity of the isolates was evaluated in two independent experiments under identical experimental conditions with seeds artificially inoculated by immersion in conidial suspensions. In both experiments, inoculation of the maize kernels had no or only a small effect on plant stand. Only inoculation with F. subglutinans (I) caused a significant reduction in the number of plants per seed tray by 10–20% in both experiments (Fig. 1). More significant differences were observed in the plant dry weight. Both isolates of F. verticillioides and isolate F. subglutinans (I) reduced the plant dry weight by around 50–60%. The isolates F. subglutinans (II), F. subglutinans (III) and F. cerealis were intermediate in pathogenicity, whereas F. proliferatum and F. poae had no effect or were only mildly pathogenic (Fig. 2).
Effect of seed inoculation with conidia of different isolates of Fusarium on number of maize seedlings (Means of 2 individual experiments, each with 4 seed trays per treatment and 25 seeds per tray; determined 14 days after sowing). Different letters above bars indicate statistically significant differences between treatments within the experiments (p ≤ 0.05)
Effect of seed inoculation with conidia of different isolates of Fusarium on biomass of maize seedlings (Means of 2 individual experiments, each with 4 seed trays per treatment and 25 seeds per tray; determined 14 days after sowing). Different letters above bars indicate statistically significant differences between treatments within the experiments (p ≤ 0.05)
The differential impact on plant stand as compared to plant biomass was also seen in the second set of experiments. Again, plant biomass was more affected than plant number (Figs. 3 and 4). Inoculation with 107 conidia per ml of F. subglutinans (I) or F. subglutinans (II) and 107 and 108 conidia per ml of F. verticillioides (I) significantly reduced the plant biomass. Inoculation with conidial concentrations of 105 and 106 per ml of F. verticillioides (I) also caused a reduction in plant biomass that was statistically significant only in one case.
Effect of seed inoculation with different concentrations of conidial suspensions of Fusarium verticillioides (I) and two isolates of F. subglutinans (1 × 107 conidia per ml) on number of maize seedlings (Means of 2 individual experiments, each with 4 seed trays per treatment and 25 seeds per tray; determined 14 days after sowing). Different letters above bars indicate statistically significant differences between treatments within the experiments (p ≤ 0.05)
Effect of seed inoculation with different concentrations of conidial suspensions of Fusarium verticillioides (I) and two isolates of F. subglutinans (1 × 107 conidia per ml) on biomass of maize seedlings (Means of 2 individual experiments, each with 4 seed trays per treatment and 25 seeds per tray; determined 14 days after sowing). Different letters above bars indicate statistically significant differences between treatments within the experiments (p ≤ 0.05)
After sowing of seeds that had been stored after inoculation for 80 days at 4 °C, the plant biomass was similarly reduced as in the case of plants grown from seeds sown 1 day after inoculation (Fig. 5), indicating that storage of inoculated seeds had no adverse effect on the virulence of the inoculum. This also implies that in such trials a stock of inoculated seeds can be prepared in advance for use in several experiments.
Effect of storage of maize seed inoculated with Fusarium verticillioides (II) (1 × 107 conidia per ml) on plant stand (solid bars) and plant dry weight (hatched bars). Storage duration after inoculation was 80 days. Included in the experiment were non-inoculated seeds and seeds sown 1 day after inoculation (dpi) (Means of 3 seed trays per treatment and 25 seeds per tray; determined 14 days after sowing). Different letters above bars indicate statistically significant differences between treatments (p ≤ 0.05)
In the experiments evaluating the potential of microorganisms to protect against seed-borne F. subglutinans (I), inoculation of maize seed with the pathogen only reduced the biomass of the seedlings growing from this seed by about 30% (Fig. 6). No reduction was recorded after seed treatment with the chemical fungicide Maxim XL and some of the tested microorganisms. Among the latter, the level of control was highest by the necrotrophic mycoparasite Clonostachys rosea IK726. Strain Clonostachys rosea HJS1881 was similarly effective. Of the six strains of Chaetomium included in our study, C. cochliodes, C. globosum and C. ramosissimum were the most effective. A certain level of control was also achieved with the non-pathogenic strain F. oxysporum MSA 35 and F. solani BBA 64531. For strains Streptomyces antimycoticus FZB 53, Trichoderma asperellum T23 and T. harzianum T16 anti-Fusarium activity has been reported before (compare Table 1). The microorganism that failed to provide protection included, among others, two strains of the bacterium Lysobacter enzymogenes and the ascomycete Persiciospora moreaui.
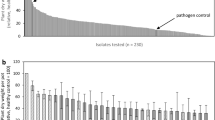
Efficacy of treatment of maize kernels with different bacteria and fungi against seed-inoculated Fusarium subglutinans (I). Pooled results from altogether 8 experiments. Means and standard deviation of plant dry weight from 2 seed trays, each with 25 seeds each per treatment, in relation to the controls in the respective experiments. The treatments were tested once (solid bars) or twice (hatched bars). The chemical standard seed treatment Maxim XL, a healthy control and a pathogen control were included in all eight experiments (dotted bars)
Inoculation of the potting substrate with fusaria caused a significant reduction in the plant biomass. Isolates F. poae, F. culmorum Fu13 and F. avenaceum To8 reduced the biomass by about half, whereas in potting substrate inoculated with strains F. culmorum VIII18 and F. graminearum Ck3 and Os12 germination was completely inhibited (Fig. 7).
Effect of inoculation of the potting substrate with different fusaria on biomass of maize seedlings (Inoculum: pearl barley, inoculum concentration: 3% (w/v). Means of 5 pots with 6 seeds each per treatment; determined 15 days after sowing). Different letters above bars indicate statistically significant differences (p ≤ 0.05)
The millet, buckwheat and pearl barley inocula all reduced the plant stand (not shown) and plant biomass in a dose-dependent manner (Fig. 8). However, the results obtained with the millet inoculum were less variable, and the millet inoculum appeared to cause a greater reduction in biomass than the buckwheat and pearl barley inocula.
Effect of inoculation of the potting substrate with F. culmorum VIII18 raised on different substrates on biomass of maize seedlings. In Experiment 2, two of the treatments included a seed treatment with Thiram. (Means of 3 pots with 6 seeds each per treatment; determined 14 days after sowing). Different letters above bars indicate statistically significant differences (p ≤ 0.05)
Compared to untreated seed, treatment with the chemical Thiram reduced the loss caused by F. culmorum significantly. Based on biomass, the level of control by Thiram was about 60% and 45% in potting substrate with 1 and 3% inoculum, respectively.
Discussion
In this study, fusaria were isolated from maize kernels and their pathogenicity was determined in tests in seed trays. Because the kernels were surface-disinfected before placement on filter paper, it is safe to assume that the isolated fusaria were located in the seed coat or deeper. The species isolated, F. verticillioides, F. subglutinans, F. cerealis and F. poae are all known pathogens of maize causing seedling blight and rots of roots, stalks and cobs (Leslie and Summerell 2006, Dorn et al. 2011, Solorzano and Malvick 2011, Oldenburg et al. 2017, Gromadzka et al. 2019).
In the pathogenicity tests, differences between isolates were nevertheless observed. For example, isolates of F. verticillioides and F. subglutinans reduced the plant dry weight significantly, whereas the tested isolates of F. proliferatum and F. poae had only a weak effect. However, due to the low number of isolates employed conclusions regarding the potential of the species to affect germination and to cause seedling diseases are difficult to draw. In a similar study with two isolates each of the species F. graminearum, F. verticillioides, F. oxysporum, F. proliferatum, F. solani and F. subglutinans, most of the isolates were pathogenic to maize seedlings, but a few were not, and aggressiveness of the isolates varied as much within a species as among species (Munkvold and O`Mara 2002). In experiments including different maize varieties and isolates of F. verticillioides (reported as F. moniliforme), the latter differed in aggressiveness to corn seedlings, but there was no apparent isolate x cultivar interaction, which indicated a lack of physiological specialization of F. verticillioides (Bacon et al. 1994).
Studies with artificial inoculation of seeds of crops other than maize with fusaria commonly used conidial concentrations of 1 × 105–1 × 106 per ml (Wilke et al. 2007; Sousa et al. 2008; Imathiu et al. 2010; Maitlo et al. 2016). For the inoculation of maize kernels with F. verticillioides, use of concentrations of 1 × 105 (reported as F. moniliforme; Galperin et al. 2003) and 1 × 107 conidia per ml, respectively (Pereira et al. 2011) has been reported. In our experiments, concentrations of 1 × 107 and 1 × 108 conidia per ml of F. verticillioides caused a higher loss of biomass than 1 × 105 and 1 x 106 conidia per ml. This deviates from a study reporting that identical results were obtained with a wide range of conidial concentrations of F. verticillioides. However, the authors reason that mycelia present in the conidial suspensions may have contributed to this result (Bacon et al. 1994, Bacon and Hinton 1996).
Large differences in plant development were also seen when healthy maize kernels were sown in potting substrate inoculated with different fusaria. At the inoculum concentration of 3%, one isolate of F. culmorum (isolated from wheat kernels) and two of F. graminearum (from maize silage) completely halted plant growth. In two follow-up experiments, the loss caused by the same inoculum concentration was lower and could be partially compensated by seed treatment with a chemical fungicide. Of the three substrates tested for the production of inoculum, millet seeds appeared to be preferable. Taken together, the results indicate that the chosen experimental setup is suited also for screening seed-applied microbial antagonists for activity against soil-borne fusaria. However, as with other soil-borne pathosystems, maintaining an adequate infection pressure in consecutive experiments is critical for obtaining reproducible results.
In the experiments testing the potential of microorganisms to control seed-borne Fusarium infections, we used inoculation by immersion of the seeds in mixtures of the biocontrol agents and conidia of the pathogen, since sequential application could have let to washing off the inoculum applied first. Co-inoculation of pathogen and antagonist has been also used in other studies (e.g. Pereira et al. 2011). Several reports describe Clonostachys rosea as a fungal antagonist with activity against different plant pathogens (Cota et al. 2009; Hue et al. 2009; Rodríguez et al. 2011). Likewise, we also recorded a high level of control of F. subglutinans by the two isolates of C. rosea included in our study. Clonostachys rosea IK726 is known as an antagonist of a number of seed-borne fungal pathogens including Fusarium culmorum, Bipolaris sorokiniana and Alternaria spp. (Knudsen et al. 1995; Jensen et al. 2007; Koch et al. 2010; Nygren et al. 2018). Also among the best performing isolates were three isolates of Chaetomium, i.e., C. globosum, C. cochliodes and C. ramosissimum. The saprophytic genus Chaetomium comprises a large number of species, some of which are antagonists of plant pathogens (Madbouly and Abdel-Wareth 2020). For C. globosum, it has been reported that the ascospores germinated rapidly and covered the seed coat with a dense mycelium (Hubbard et al. 1982). Surprisingly, seed inoculation with Fusarium solani and F. oxysporum MSA also provided protection against co-applied F. subglutinans. The significance of this finding remains to be elucidated in further studies. At present, one can only speculate that mechanisms similar to those responsible for the protection against Fusarium wilt by non-pathogenic strains of F. oxysporum (Alabouvette et al. 2009) may be involved.
Altogether, it appears that some of the tested strains are potentially suited as seed treatments to protect maize against seed-borne fusaria. However, after artificial inoculation the pathogen inoculum is located on the seed surface where it is more exposed to control agents than deeper-seated infections, an important limitation when correlating the results from artificial inoculation with the practical field conditions (Uoti 1979). With this in mind, the results obtained are preliminary and require verification with larger sample numbers and under conditions more closely resembling the agricultural practice, including use of naturally infected seeds. Since seed treatments must control both, seed- and soil-borne fusaria, future work should also include testing against pathogen attack from the soil. For this, the test method developed in this work using inoculation of the potting substrate with fusaria appears suitable.
References
Alabouvette C, Olivain C, Migheli Q, Steinberg C (2009) Microbiological control of soil-borne phytopathogenic fungi with special emphasis on wilt-inducing Fusarium oxysporum. New Phytol 184:529–544
Bacon CW, Hinton DM (1996) Symptomless endophytic colonization of maize by Fusarium moniliforme. Can J Bot 74:1195–1202
Bacon CW, Hinton DM (2007) Potential for control of seedling blight of wheat caused by Fusarium graminearum and related species using the bacterial endophyte Bacillus mojavensis. Biocontrol Sci Technol 17:81–94
Bacon CW, Hinton DM, Richardson MD (1994) A corn seedling assay for resistance to Fusarium moniliforme. Plant Dis 78:302–305
Bressan W, Figueiredo JEF (2005) Biological control of Stenocarpella maydis in maize seed with antagonistic Streptomyces sp. isolates. J Phytopathol 153:623–626
Browne RA, Cooke BM (2005) Resistance of wheat to Fusarium spp. in an in vitro seed germination assay and preliminary investigations into the relationship with Fusarium head blight resistance. Euphytica 141:23–32
Cota LV, Maffia LA, Mizubuti ESG, Macedo PEF (2009) Biological control by Clonostachys rosea as a key component in the integrated management of strawberry gray mold. Biol Control 50:222–230
Dorn B, Forrer HR, Jenny E, Wettstein FE, Bucheli TD, Vogelgsang S (2011) Fusarium species complex and mycotoxins in grain maize from maize hybrid trials and from grower’s fields. J Appl Microbiol 111:693–706
El-Hasan A, Schöne J, Höglinger B, Walker F, Voegele RT (2018) Assessment of the antifungal activity of selected biocontrol agents and their secondary metabolites against Fusarium graminearum. Eur J Plant Pathol 150:91–103
Galperin M, Graf S, Kenigsbuch D (2003) Seed treatment prevents vertical transmission of Fusarium moniliforme, making a significant contribution to disease control. Phytoparasitica 31:344–352
Gilardi G, Tinivella F, Gullino ML, Garibaldi A (2005) Seed dressing to control Fusarium oxysporum f.sp. lactucae. J Plant Dis Prot 112:240–246
Gromadzka K, Chełkowski J, Basińska-Barczak A, Lalak-Kańczugowska J (2019) Diversity and mycotoxin production by Fusarium temperatum and Fusarium subglutinans as causal agents of pre-harvest Fusarium maize ear rot in Poland. J Appl Genet 60:113–121
Hörmann V, Gossmann M, Junge H, Büttner C (2010) Morphologische Charakterisierung von Persiciospora moreaui und Melanospora zamiae in Fusarium spp.-Isolaten von Spargel- und Gurkenpflanzen. Julius-Kuhn-Arch 428:412
Hubbard JP, Harman GE, Eckenrode CJ (1982) Interaction of a biological control agent, Chaetomium globosum, with seed coat microflora. Can J Microbiol 28:431–437
Hue AG, Voldeng HD, Savard ME, Fedak G, Tian X, Hsiang T (2009) Biological control of Fusarium head blight of wheat with Clonostachys rosea strain ACM941. Can J Plant Pathol 31:169–179
Imathiu SM, Hare MC, Ray RV, Back M, Edwards SG (2010) Evaluation of pathogenicity and aggressiveness of F. langsethiae on oat and wheat seedlings relative to known seedling blight pathogens. Eur J Plant Pathol 126:203–216
Jensen DF, Knudsen IM, Mamarabadi M, Hockenhull J, Jensen B (2007) Development of a biocontrol agent for plant disease control with special emphasis on the near commercial fungal antagonist Clonostachys rosea strain ‘IK726’. Australas Plant Pathol 36:95–101
Johnsson L, Hökeberg M, Gerhardson B (1998) Performance of the Pseudomonas chlororaphis biocontrol agent MA 342 against cereal seed-borne diseases in field experiments. Eur J Plant Pathol 104:701–711
Knudsen MB, Hockenhull J, Jensen DF (1995) Biocontrol of seedling diseases of barley and wheat caused by Fusarium culmorum and Bipolaris sorokiniana: effects of selected fungal antagonists on growth and yield components. Plant Pathol 44:467–477
Koch E (1997) Screening of rhizobacteria for antagonistic activity against P. ultimum on cucumber and kale. J Plant Dis Prot 104:353–361
Koch E, Löffler I (2009) Partial characterization of the antimicrobial activity of Streptomyces antimycoticus FZB53. J Plant Dis Prot 157:235–242
Koch E, Roberts SJ (2014) Non-chemical seed treatment in the control of seed-borne pathogens. In: Gullino M, Munkvold G (eds) Global perspectives on the health of seeds and plant propagation material. plant pathology in the 21st century (contributions to the 9th international congress), vol 6. Springer, Dordrecht
Koch E, Kempf HJ, Hessenmüller A (1998) Characterization of the biocontrol activity and evaluation of potential growth-promoting properties of selected rhizobacteria. J Plant Dis Prot 105:567–580
Koch E, Schmitt A, Stephan D et al (2010) Evaluation of non-chemical seed treatment methods for the control of Alternaria dauci and A. radicina on carrot seeds. Eur J Plant Pathol 127:99–112
Leslie JF, Summerell BA (2006) The Fusarium laboratory manual. Blackwell, Oxford
Madbouly AK, Abdel-Wareth MT (2020) The use of Chaetomium taxa as biocontrol agents. In: Abdel-Azeem AM (ed) Recent developments on genus Chaetomium. Springer, Cham, pp 251–266
Maitlo SA, Syed RN, Rustamani MA, Khuhro RD, Lodhi AM (2016) Influence of inoculation methods and inoculum levels on the aggressiveness of Fusarium oxysporum f. sp. ciceris on chickpea and plant growth. Int J Agric Biol 18:31–36
Mancini V, Romanazzi G (2014) Seed treatments to control seedborne fungal pathogens of vegetable crops. Pest Manag Sci 70:860–868
Mao W, Lumsden RD, Lewis JA, Hebbar PK (1998) Seed treatment using pre-infiltration and biocontrol agents to reduce damping-off of corn caused by species of Pythium and Fusarium. Plant Dis 82:294–299
Moussart A, Tivoli B, Lemarchand E, Deneufbourg F, Roi S, Sicard G (1998) Role of seed infection by the Ascochyta blight pathogen of dried pea (Mycosphaerella pinodes) in seedling emergence, early disease development and transmission of the disease to aerial plant parts. Eur J Plant Pathol 104:93–102
Mulè G, Susca A, Stea G, Moretti A (2004a) A species-specific PCR assay based on the calmodulin partial gene for identification of Fusarium verticillioides, F. proliferatum and F. subglutinans. Eur J Plant Pathol 110:495–502
Mulè G, Susca A, Stea G, Moretti A (2004b) Specific detection of the toxigenic species Fusarium proliferatum and F. oxysporum from asparagus plants using primers based on calmodulin gene sequences. FEMS Microbiol Lett 230:235–240
Munkvold GP, O’Mara JK (2002) Laboratory and growth chamber evaluation of fungicidal seed treatments for maize seedling blight caused by Fusarium species. Plant Dis 86:143–150
Munkvold GP, White DG (eds) (2016) Compendium of corn diseases (p. 165). APS Press, St. Paul, Minnesota
Nagy E, Moldovan V (2007) The effect of fungicide treatments on wheat common bunt (Tilletia spp.) in Transylvania. Rom Agric Res 24:33–38
Nicholson P, Simpson DR, Weston G, Rezanoor HN, Lees AK, Parry DW, Joyce D (1998) Detection and quantification of Fusarium culmorum and Fusarium graminearum in cereals using PCR assays. Physiol Mol Plant P 53:17–37
Nielsen J (1976) A method for artificial inoculation of oats and barley for seed treatment trials on seedling-infecting smuts. Can Plant Dis Surv 56:114–116
Nygren K, Dubey M, Zapparata A, Iqbal M, Tzelepis GD, Durling MB, Hensen DF, Karlsson M (2018) The mycoparasitic fungus Clonostachys rosea responds with both common and specific gene expression during interspecific interactions with fungal prey. Evol Appl 11:931–949
O`Callaghan M (2016) Microbial inoculation of seed for improved crop performance: issues and opportunities. Appl Microbiol Biotechnol 100:5729–5746
Oldenburg E, Höppner F, Ellner F, Weinert J (2017) Fusarium diseases of maize associated with mycotoxin contamination of agricultural products intended to be used for food and feed. Mycotoxin Res 33:167–182
Pandey A, Palni LMS, Hebbar KP (2001) Suppression of damping-off in maize seedlings by Pseudomonas corrugata. Microbiol Res 156:191–194
Parry DW, Nicholson P (1996) Development of a PCR assay to detect Fusarium poae in wheat. Plant Pathol 45:383–391
Patiño B, Mirete S, González-Jaén MT, Mulé G, Rodríguez MT, Vázquez C (2004) PCR detection assay of fumonisin-producing Fusarium verticillioides strains. J Food Prot 67:1278–1283
Pereira P, Ibáñez SG, Agostini E, Etcheverry M (2011) Effects of maize inoculation with Fusarium verticillioides and with two bacterial biocontrol agents on seedlings growth and antioxidative enzymatic activities. Appl Soil Ecol 51:52–59
Rodríguez MA, Cabrera G, Gozzo FC, Eberlin MN, Godeas A (2011) Clonostachys rosea BAFC3874 as a Sclerotinia sclerotiorum antagonist: mechanisms involved and potential as a biocontrol agent. J Appl Microbiol 110:1177–1186
Solorzano CD, Malvick DK (2011) Effects of fungicide seed treatments on germination, population, and yield of maize grown from seed infected with fungal pathogens. Field Crop Res 122:173–178
Sousa MV, Machado JC, Pfenning LH, Kawasaki VH, Araújo DV, Silva AA, Martini Neto A (2008) Métodos de inoculação e efeitos de Fusarium oxysporum f. sp. vasinfectum em sementes de algodoeiro. Trop Plant Pathol 33:41–48
Turner AS, Lees AK, Rezanoor HN, Nicholson P (1998) Refinement of PCR-detection of Fusarium avenaceum and evidence from DNA marker studies for phenetic relatedness to Fusarium tricinctum. Plant Pathol 47:278–288
Uoti J (1979) Study of control of seed-borne Fusarium in cereals. Ann Agr Fenn 18:149–153
White TJ, Bruns T, Lee S, Taylor JW (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ (eds) PCR protocols: a guide to methods and applications. Academic Press, New York, pp 315–322
Wilke AL, Bronson CR, Tomas A, Munkvold GP (2007) Seed transmission of Fusarium verticillioides in maize plants grown under three different temperature regimes. Plant Dis 91:1109–1152
Acknowledgements
Open Access funding provided by Projekt DEAL. Thanks are due to Dan Funck Jensen (Swedish Agricultural University, Uppsala), Monika Gossman (formerly Humboldt University Berlin), Hans-Josef Schroers (Agricultural Institute of Slovenia), Abbas El-Hasan (University of Hohenheim, Institute of Phytomedicine), Federico Tinivella (University of Turin) and Wolfgang Maier (JKI, Institute for Epidemiology and Pathogen Diagnostics) for supplying fungal strains.
Funding
The research presented here was for the most part carried out in a joint project between the JKI Institute for Biological Control and Fraunhofer-Institut für Organische Elektronik, Elektronenstrahl- und Plasmatechnik FEP, Dresden. A part of this work was supported by funds of the Federal Ministry of Food and Agriculture (BMEL) based on a decision of the parliament of the Federal Republic of Germany via the Federal Office for Agriculture and Food (BLE).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Koch, E., Zink, P., Pfeiffer, T. et al. Artificial inoculation methods for testing microorganisms as control agents of seed- and soil-borne Fusarium-seedling blight of maize. J Plant Dis Prot 127, 883–893 (2020). https://doi.org/10.1007/s41348-020-00350-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41348-020-00350-w