Abstract
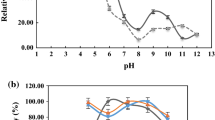
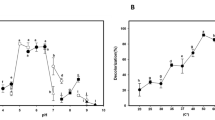
Biocatalytic decolorization of azo dyes is hampered by their recalcitrance and the characteristics of textile effluents. Alkaline pH and heavy metals present in colored wastewaters generally limit the activity of enzymes such as laccases of fungal origin; this has led to an increasing interest in bacterial laccases. In this work, the dye decolorization ability of LAC_2.9, a laccase from the thermophilic bacterial strain Thermus sp. 2.9, was investigated. Its resistance towards different pHs and toxic heavy metals frequently present in wastewaters was also characterized. LAC_2.9 was active and highly stable in the pH range of 5.0 to 9.0. Even at 100 mM Cd+2, As+5 and Ni+2 LAC_2.9 retained 99%, 86% and 75% of its activity, respectively. LAC_2.9 was capable of decolorizing 98% of Xylidine, 54% of RBBR, 40% of Gentian Violet, and 33% of Methyl Orange after 24 h incubation at pH 9, at 60 °C, without the addition of redox mediators. At acidic pH, the presence of the mediator 1-hydroxybenzotriazole generally increased the catalytic effectiveness. We analyzed the degradation products of laccase-treated Xylidine and Methyl Orange by capillary electrophoresis and mass spectrometry, and propose a degradation pathway for these dyes. For its ability to decolorize recalcitrant dyes, at pH 9, and its stability under the tested conditions, LAC_2.9 could be effectively used to decolorize azo dyes in alkaline and heavy metal containing effluents.





Similar content being viewed by others
Abbreviations
- RBBR:
-
Remazol Brilliant Blue R
- HBT:
-
1-Hydroxybenzotriazole
- ABTS:
-
2,2′-Azino-di-[3-ethylbenzthiazoline sulfonate]
- pHBA:
-
Para-hydroxybenzoic acid
- CE:
-
Capillary electrophoresis
References
Adedayo O, Javadpour S, Taylor C, Anderson WA, Moo-Young M (2004) Decolourisation and detoxification of methyl red by aerobic bacteria from a wastewater treatment plant. J Microbiol Biotechnol 20:545–550. https://doi.org/10.1023/b.wibi.0000043150.37318.5f
Adnan LA, Sathishkumar P, Mohd Yusoff AR, Hadibarata T (2015) Metabolites characterisation of laccase mediated Reactive Black 5 biodegradation by fast growing ascomycete fungus Trichoderma atroviride F03. Int Biodeterior Biodegrad 104:274–282. https://doi.org/10.1016/j.ibiod.2015.05.019
Almansa E, Kandelbauer A, Pereira L, Cavaco-Paulo A, Gubitz GM (2004) Influence of structure on dye degradation with laccase mediator systems. Biocatal Biotransform 22:315–324. https://doi.org/10.1080/10242420400024508
Anastasi A, Parato B, Spina F, Tigini V, Prigione V, Varese GC (2011) Decolourisation and detoxification in the fungal treatment of textile wastewaters from dyeing processes. N Biotechnol 29:38–45. https://doi.org/10.1016/j.nbt.2011.08.006
Blánquez A, Rodríguez J, Brissos V, Mendes S, Martins LO, Ball AS, Arias ME, Hernández M (2019) Decolorization and detoxification of textile dyes using a versatile Streptomyces laccase-natural mediator system. Saudi J Biol Sci 26:913–920. https://doi.org/10.1016/j.sjbs.2018.05.020
Burriel Marti F, Lucena Conde F, Arribas Jimeno S, Hernández Méndez J (2002) Química analítica cualitativa. International Thomson Editores Spain, Madrid
Ciullini I, Tilli S, Scozzafava A, Briganti F (2008) Fungal laccase, cellobiose dehydrogenase, and chemical mediators: combined actions for the decolorization of different classes of textile dyes. Bioresour Technol 99:7003–7010. https://doi.org/10.1016/j.biortech.2008.01.019
Collivignarelli M, Abbà A, Carnevale Miino M, Damiani S (2019) Treatments for color removal from wastewater: state of the art. J Environ Manag 236:727–745. https://doi.org/10.1016/j.jenvman.2018.11.094
Di Rienzo JA, Casanoves F, Balzarini MG, Gonzalez L, Tablada M, Robledo CW (2017) InfoStat versión 2017. Grupo InfoStat, FCA, Universidad Nacional de Córdoba, Argentina. http://www.infostat.com.ar
Du LN, Li G, Xu FC, Pan X, Wen LN, Wang Y (2014) Rapid decolorization of methyl orange by a novel Aeromonas sp. strain DH-6. Water Sci Technol 69:2004–2013. https://doi.org/10.2166/wst.2014.099
Enayatzamir K, Tabandeh F, Yakhchali B, Alikhani HA, Rodríguez Couto S (2009) Assessment of the joint effect of laccase and cellobiose dehydrogenase on the decolouration of different synthetic dyes. J Hazard Mater 169:176–181. https://doi.org/10.1016/j.jhazmat.2009.03.088
Galai S, Korri-Youssoufi H, Marzouki MN (2014) Characterization of yellow bacterial laccase SmLac/role of redox mediators in azo dye decolorization. J Chem Technol Biotechnol 89:1741–1750. https://doi.org/10.1002/jctb.4254
Garg SK, Tripathi M (2016) Microbial strategies for discoloration and detoxification of azo dyes from textile effluents. Res J Microbiol 12:1–19. https://doi.org/10.3923/jm.2017.1.19
Guan ZB, Luo Q, Wang HR, Chen Y, Liao XR (2018) Bacterial laccases: promising biological green tools for industrial applications. Cell Mol Life Sci 75:3569. https://doi.org/10.1007/s00018-018-2883-z
Iark D, dos Reis Buzzo AJ, Garcia JAA, Côrrea VG, Helm CV, Corrêa RCG, Peralta RA, Moreira RFPM, Bracht A, Peralta RM (2019) Enzymatic degradation and detoxification of azo dye Congo red by a new laccase from Oudemansiella canarii. Bioresour Technol 289:121655. https://doi.org/10.1016/j.biortech.2019.121655
Kalme S, Ghodake G, Govindwar S (2007) Red HE7B degradation using desulfonation by Pseudomonas desmolyticum NCIM 2112. Int Biodeterior Biodegrad 60:327–333. https://doi.org/10.1016/j.ibiod.2007.05.006
Kalme S, Jadhav S, Jadhav M, Govindwar S (2009) Textile dye degrading laccase from Pseudomonas desmolyticum NCIM 2112. Enzyme Microb Technol 44:65–71. https://doi.org/10.1016/j.enzmictec.2008.10.005
Kim Y, Nicell JA (2006) Impact of reaction conditions on the laccase catalyzed conversion of bisphenol A. Bioresour Technol 197:1431–1442. https://doi.org/10.1016/j.biortech
Kokol V, Doliška A, Eichlerová I, Baldrian P, Nerud F (2007) Decolorization of textile dyes by whole cultures of Ischnoderma resinosum and by purified laccase and Mn-peroxidase. Enzyme Microb Technol 40:1673–1677. https://doi.org/10.1016/j.enzmictec.2006.08.015
Lade H, Kadam A, Paul D, Govindwar S (2015) Biodegradation and detoxification of textile azo dyes by bacterial consortium under sequential microaerophilic/aerobic processes. EXCLI J 14:158–174. https://doi.org/10.17179/excli2014-642
Legerská B, Chmelová D, Ondrejovič M (2018) Decolourization and detoxification of monoazo dyes by laccase from the white-rot fungus Trametes versicolor. J Biotechnol 285:84–90. https://doi.org/10.1016/jjbiotec.2018.08.011
Levin L, Grassi E, Carballo R (2012) Efficient azoic dye degradation by Trametes trogii and a novel strategy to evaluate products released. Int Biodeterior Biodegrad 75:214–222. https://doi.org/10.1016/j.ibiod.2012.10.005
Liu H, Cheng Y, Du B, Tong C, Liang S, Han S, Zheng S, Lin Y (2015) Overexpression of a novel thermostable and chloride-tolerant laccase from Thermus thermophilus SG0.5JP17-16 in Pichia pastoris and its application in synthetic dye decolorization. PLoS One 10(3):e0119833. https://doi.org/10.1371/journal.pone.0119833
Lončar N, Božić N, Lopez-Santin J, Vujčić Z (2013) Bacillus amyloliquefaciens laccase—from soil bacteria to recombinant enzyme for wastewater decolorization. Bioresour Technol 147:177–183. https://doi.org/10.1016/j.biortech.2013.08.056
Ma S, Liu N, Jia H, Dai D, Zang J, Cao Z, Dong J (2018) Expression, purification, and characterization of a novel laccase from Setosphaeria turcica in Escherichia coli. J Basic Microbiol 58:68–75. https://doi.org/10.1002/jobm.201700212
Mandic M, Djokic L, Nikolaivits E, Prodanovic R, O’Connor K, Jeremic S, Topakas E, Nikodinovic-Runic J (2019) Identification and characterization of new laccase biocatalysts from Pseudomonas species suitable for degradation of synthetic textile dyes. Catalysts 9:629. https://doi.org/10.3390/catal9070629
Mishra A, Kumar S, Pandey A (2011) Laccase production and simultaneous decolorization of synthetic dyes in unique inexpensive medium by new isolates of white rot fungus. Int Biodeterior Biodegrad 65:487–493. https://doi.org/10.1016/j.ibiod.2011.01.011
Molina-Guijarro JM, Pérez J, Muñoz-Dorado J, Guillén F, Moya R, Hernández M, Arias ME (2009) Detoxification of azo dyes by a novel pH-versatile, salt-resistant laccase from Streptomyces ipomoea. Int Microbiol 12:13–21. https://doi.org/10.2436/20.1501.01.77
Mongay C, Cerdà V (1974) A Britton–Robinson buffer of known ionic strength. Ann Chim 64:409–412
Moya R, Hernández M, García-Martín AB, Ball AS, Arias ME (2010) Contributions to a better comprehension of redox-mediated decolouration and detoxification of azo dyes by a laccase produced by Streptomyces cyaneus CECT 3335. Bioresour Technol 101:2224–2229. https://doi.org/10.1016/j.biortech.2009.11.061
Navas LE, Martínez FD, Taverna ME, Fetherolf MM, Eltis LD, Nicolau V, Estenoz D, Campos E, Benintende GB, Berretta MF (2019) A thermostable laccase from Thermus sp. 2.9 and its potential for delignification of Eucalyptus biomass. AMB Express 9(1):24. https://doi.org/10.1186/s13568-019-0748-y
Parshetti GK, Kalme SD, Saratale GD, Govindwar SP (2006) Biodegradation of malachite green by Kocuria rosea MTCC 1532. Acta Chim Slov 53:492–498
Pereira L, Coelho AV, Viegas CA, dos Santos MMC, Robalo MP, Martins LO (2009) Enzymatic biotransformation of the azo dye Sudan Orange G with bacterial CotA-laccase. J Biotechnol 139:68–77. https://doi.org/10.1016/j.jbiotec.2008.09.001
Si J, Peng F, Cui B (2013) Purification, biochemical characterization and dye decolorization capacity of an alkali-resistant and metal-tolerant laccase from Trametes pubescens. Bioresour Technol 128:49–57. https://doi.org/10.1016/j.biortech.2012.10.085
Singh RL, Singh PK, Singh RP (2015) Enzymatic decolorization and degradation of azo dyes-a review. Int Biodeterior Biodegrad 104:21–31. https://doi.org/10.1016/j.ibiod.2015.04.027
Takeda S, Tanaka Y, Nishimura Y, Yamane M, Siroma Z, Wakida S (1999) Analysis of dyestuff degradation products by capillary electrophoresis. J Chromatogr A 853:503–509. https://doi.org/10.1016/s0021-9673(99)00579-8
Telke AA, Kadam AA, Jagatap SS, Jadhav JP, Govindwar SP (2010) Biochemical characterization and potential for textile dye degradation of blue laccase from Aspergillus ochraceus NCIM-1146. Biotechnol Bioprocess Eng 15:696–703. https://doi.org/10.1007/s12257-009-3126-9
Thakur JK, Paul S, Dureja P, Annapurna K, Padaria JC, Gopal M (2014) Degradation of sulphonated azo dye Red HE7B by Bacillus sp. and elucidation of degradative pathways. Curr Microbiol 69:183–191. https://doi.org/10.1007/s00284-014-0571-2
Torres E, Bustos-Jaimes I, Borgne SL (2003) Potential use of oxidative enzymes for the detoxification of organic pollutants. Appl Catal B-Environ 46:1–15. https://doi.org/10.1016/s0926-3373(03)00228-5
Unuofin JO, Okoh AI, Nwodo UU (2019) Aptitude of oxidative enzymes for treatment of wastewater pollutants: a laccase perspective. Molecules 24:2064. https://doi.org/10.3390/molecules24112064
Verma A, Shirkot P (2014) Purification and characterization of thermostable laccase from thermophilic Geobacillus thermocatenulatus MS5 and its applications in removal of textile dyes. Sch Acad J Biosci 2:479–485
Xia J, Wang Q, Luo Q, Chen Y, Liao XR, Guan ZB (2019) Secretory expression and optimization of Bacillus pumilus CotA-laccase mutant GWLF in Pichia pastoris and its mechanism on Evans blue degradation. Process Biochem 20:33–41. https://doi.org/10.1016/j.procbio.2018.12.034
Zaharia C, Suteu D (2012) Textile organic dyes. Characteristics, polluting effects and separation/elimination procedures from industrial effluents. A critical overview. In: Tomasz P (ed) Organic pollutants ten years after the Stockholm Convention. Environmental and analytical update. IntechOpen. https://doi.org/10.5772/32373
Zheng F, Cui BK, Wu XJ, Meng G, Liu HX, Si J (2016) Immobilization of laccase onto chitosan beads to enhance its capability to degrade synthetic dyes. Int Biodeterior Biodegrad 110:69–78. https://doi.org/10.1016/j.ibiod.2016.03.004
Zille A, Gornacka B, Rehorek A, Cavaco-Paulo A (2005) Degradation of azo dyes by Trametes villosa laccase over long periods of oxidative conditions. Appl Environ Microbiol 71:6711–6718. https://doi.org/10.1128/aem.71.11.6711-6718.2005
Acknowledgements
We thank Irma Fuxan for the technical support in routine laboratory protocols and Dr. Morgan Fetherolf for the critical reading of the manuscript. LN was granted a fellowship from CONICET. RC, LL, and MB are staff members of CONICET. This work was supported by Instituto Nacional de Tecnología Agropecuaria (INTA) (Grant Number PNAIyAV-1130034), Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET, Argentina) and University of Buenos Aires.
Author information
Authors and Affiliations
Contributions
LN obtained and purified the enzyme and performed the decolorization experiments. RC performed the CE and MS analysis and the statistical analysis of decolorization experiments. LL and MB conceived the study and MB supervised the project. All authors have contributed to the writing and revising of the manuscript and approved the final version to be submitted.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by I. Cann.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Navas, L.E., Carballo, R., Levin, L. et al. Fast decolorization of azo dyes in alkaline solutions by a thermostable metal-tolerant bacterial laccase and proposed degradation pathways. Extremophiles 24, 705–719 (2020). https://doi.org/10.1007/s00792-020-01186-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00792-020-01186-w