- 1Department of Orthopaedics, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China
- 2Department of Orthopaedic Surgery, The First Affiliated Hospital of Zhengzhou University, Zhengzhou, China
Background: Tumor-infiltrating natural killer (NK) cells (TINKs) are crucial immune cells in tumor defense, and might be related to tumor prognosis. However, the results were discrepant among different studies. The present meta-analysis was performed to comprehensively assess the prognostic value of NK cell markers in solid tumor tissues.
Methods: PubMed, Web of Science, and EMBASE were searched to identify original researches reporting the prognostic significance of TINKs in solid tumors. NK cell markers CD56, CD57, NKp30, and NKp46 were included in the analysis. The hazard ratios (HRs) and 95% confidence intervals (CIs) of pooled overall survival (OS), disease-free survival (DFS), metastasis-free survival (MFS), progression-free survival (PFS), and recurrence-free survival (RFS) were calculated by STATA software 14.0 to assess the prognostic significance.
Results : Of the 56 included studies, there were 18 studies on CD56, 31 studies on CD57, 1 study on NKp30, and 7 studies on NKp46. High levels of CD56, CD57, NKp30, and NKp46 were significantly correlated with better OS of patients with solid malignancies (HR = 0.473, 95%CI: 0.315–0.710, p < 0.001; HR = 0.484, 95%CI: 0.380–0.616, p < 0.001; HR = 0.34, 95%CI: 0.14–0.80, p = 0.014; HR = 0.622, 95%CI: 0.470–0.821, p < 0.001, respectively). Our results also revealed that CD56, CD57, and NKp46 could act as independent prognostic predictors for favorable OS (HR = 0.372, 95%CI: 0.261–0.531, p < 0.001; HR = 0.525, 95%CI: 0.346–0.797, p = 0.003; HR = 0.559, 95%CI: 0.385–0.812, p = 0.002, respectively).
Conclusions : Our results indicated that high levels of NK cell markers in solid tumor tissues could predict favorable prognosis for solid tumor patients.
Introduction
With a high morbidity and mortality rate, malignant tumor is regarded as a significant health problem around the word, which results in heavy medical and socioeconomic burdens (1, 2). Despite substantial advances in the diagnosis and therapeutics of malignancies in recent decades, poor tumor prognosis is still an issue for researchers and clinicians (3). Therefore, more and better biomarkers are urgently needed to facilitate tumor diagnosis, predict tumor prognosis, and develop novel therapies (4).
Tumors develop and progress in a microenvironment that contains various immune cells and immune products (5). Generally, natural killer (NK) cells are considered to constitute the first line of anti-tumor defense, and provide tumor immunosurveillance, tumor lysis, and elimination of tumor metastasis (6). NK cells can surveil tumor progression by recognizing the lacking expression of major histocompatibility complex (MHC) class I molecules in tumor cells (7). A higher incidence of tumor was observed in the population with low NK cell cytotoxic activity, which further suggested that NK cells played a vital role in tumor immunosurveillance (8, 9). According to the classical NK cell activation model, NK cells directly destroy tumor cells in an MHC-unrestricted manner without the help of antigen-presenting cells (10, 11). Perforin, granzyme, and factor associated suicide ligand (FasL) pathway are all involved in NK cell-mediated tumor cell lysis (12, 13). Additionally, NK cells can release multiple soluble anti-tumor factors, such as interferon (IFN)-γ and tumor necrosis factor (TNF)-α (10, 11).
NK cells can acquire altered phenotypes and function states in the tumor microenvironment due to their plasticity (14). Although a number of tumor-infiltrating NK cells (TINKs) were detected in solid tumors, increasing evidence revealed that tumor cells might evade the immunosurveillance of NK cells. Tumor cells could downregulate the expression of activating surface receptors in NK cells, such as natural killer group 2 member d (NKG2D) (15, 16). In addition, tumor cells could not only decrease the expression of IFN-γ, TNF-α, and interleukin (IL)-2 by NK cells, but also transfer TINKs and peripheral blood (PB) NK cells (PBNKs) to an anergic or low-cytotoxic state (17). Sun et al. demonstrated that the expression of CD96 was associated with the functional exhaustion of NK cells in hepatocellular carcinoma (HCC) (18). Moreover, previous studies also showed the differentiation potentials of TINKs to a protumorigenic or proangiogenic phenotype (19, 20). Taken together, NK cells exert bidirectional effects in tumorigenesis and the explicit role of TINKs in malignancies needs to be further studied.
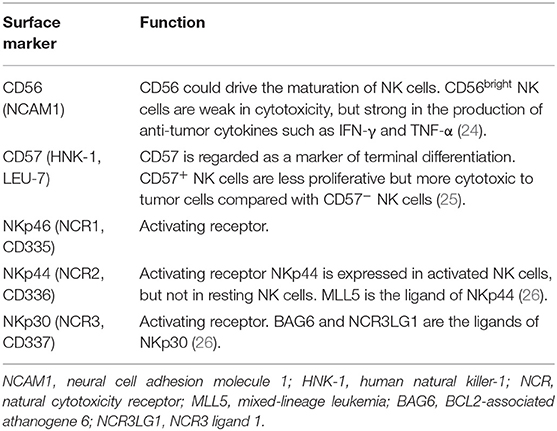
Accumulating evidence suggests that NK cells are composed of multiple sub-populations with different phenotypes and function states (21–23). A variety of surface markers have been used to label different NK cell subsets and to measure their functional properties (Table 1). In several clinical observation studies, the degree of NK cell tumor-infiltration was found to be highly associated with the prognosis of solid tumors (27, 28). However, the clinical significance of NK cells in tumor defense seems to be controversial due to previously reported discrepant results (29, 30). Therefore, the current meta-analysis was performed to comprehensively assess the prognostic value of the NK cell markers in all types of solid tumors.
Methods
Search Strategy
Two investigators (SZ and WL) independently conducted the systematic literature retrieval in PubMed, Web of Science, and EMBASE to obtain eligible researches up to October 1, 2019. The search query was “natural killer cell OR NK cell OR CD57 OR CD56 OR NKp30 OR NKp46 OR NCR OR natural cytotoxicity receptor” AND “cancer OR tumor” AND “survival OR prognosis.” Moreover, the references of relevant reviews or articles were screened for eligible studies.
Study Selection
Original research was included if it satisfied all the following criteria: (1) Studies examined the levels of NK cell markers (CD56, CD57, NKp30, and NKp46) in solid tumor tissues of patients; (2) The tumor cases were divided into two groups according to the levels of NK cell markers; (3) Studies explored the association between the levels of NK cell markers and overall survival (OS), disease-free survival (DFS), metastasis-free survival (MFS), progression-free survival (PFS), or recurrence-free survival (RFS) in solid malignancies; and (4) Sufficient data were reported in the publications for us to obtain hazard ratios (HRs) and 95% confidence intervals (CIs).
Exclusion criteria included: (1) Studies on the association between tumor prognosis and PBNKs, (2) Studies not in English, (3) Studies not on humans, and (4) Repetitive studies. If different publications reported duplicate data, only the latest one was included.
Data Extraction and Quality Assessment
Two researchers (SZ and XL) reviewed the included articles carefully and extracted the following study characteristics: author's name, year of publication, region, tumor type, clinical stage, sample size, endpoint, detection method of NK cell markers, and follow-up period. HRs and 95% CIs served as reasonable parameters in the data analysis. If the HRs and 95% CIs could not be directly obtained from the publications, the data were extracted from the Kaplan-Meier curves using Engauge Digitizer version 9.8.
The quality of the included studies were evaluated according to the Newcastle-Ottawa Scale (NOS), especially for cohort studies. NOS, ranging from 0 to 9, includes three domains: selection of the exposed cohort, comparability of the cohorts, and assessment of the outcome. Studies with a NOS score more than 6 were recognized as high-quality studies. Rating of the study quality was conducted by two independent raters (PW and BH) and disagreements were resolved by a third researcher (SC).
Data Analysis
HRs and 95% CIs were log-transformed and pooled by Stata statistical software version 14.0 (Stata Corporation, College Station, TX, USA). The heterogeneity among the research was examined by Higgins I2 statistic and Cochran Q test. A fixed-effect model was conducted if the heterogeneity was not significant (I2 < 50%); otherwise (I2 ≥ 50%), random-effect model was considered to be more suitable. The result that pooled HR was less than 1 indicated a better prognosis in the patients with a high-degree of NK cell tumor-infiltration. In addition, we evaluated the stability and credibility of summarized outcomes by sensitivity analysis and assessed the potential of publication bias by Begg's test (31). We further analyzed the prognostic significance of NK cell markers in certain types of tumors. P-values were two-sided, and P < 0.05 was considered to be statistically significant.
Results
Study Characteristics
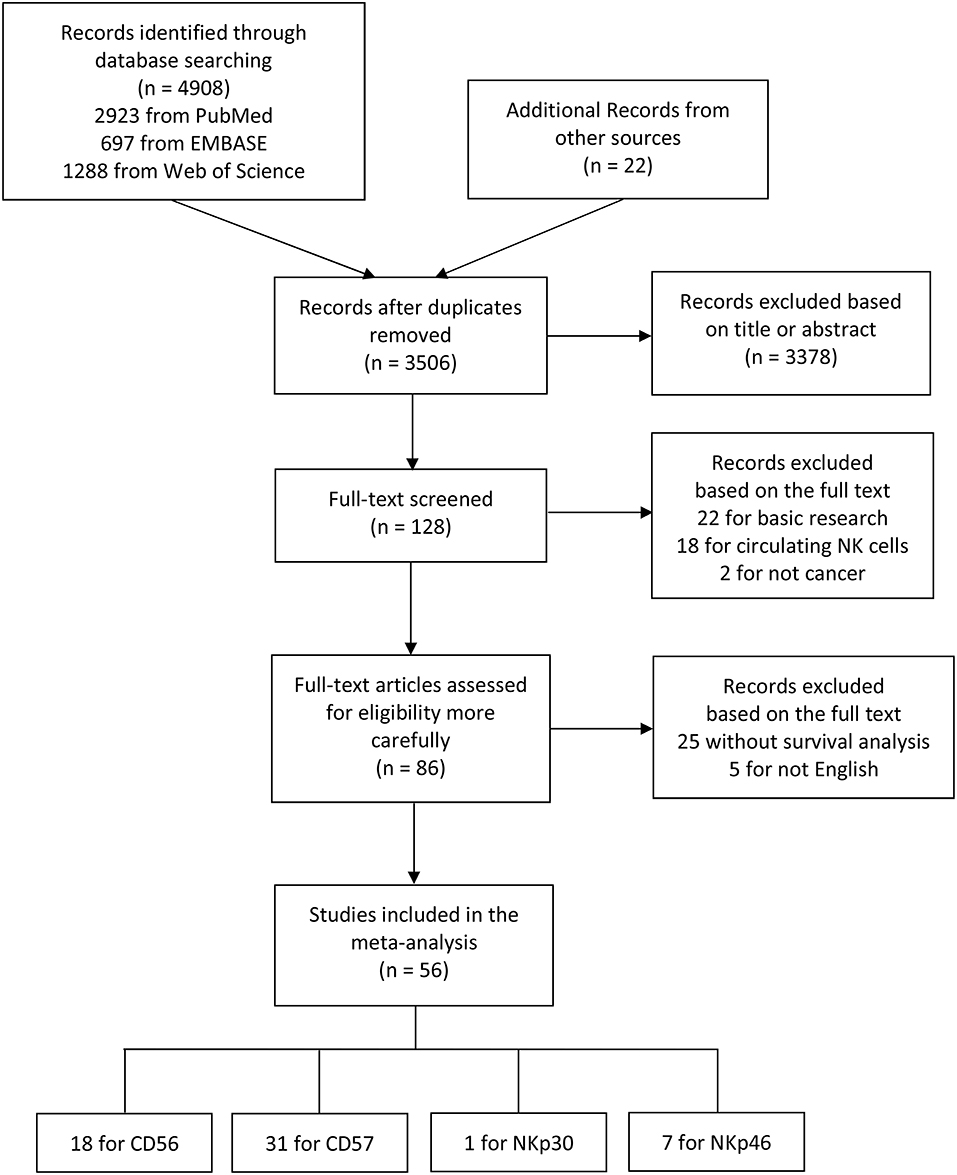
The flow diagram of literature retrieval and selection was shown in Figure 1. Among 56 included articles, there were 18 studies comprising 20 cohorts on CD56 (27, 30, 32–47), 31 studies on CD57 (29, 35, 48–76), one study on NKp30 (77), and seven studies on NKp46 (78–84). The study characteristics and NOS scores of included researches are shown in detail in Supplementary Tables 1, 2, respectively. To summarize these in brief: (1) the sample size ranges from 16 to 1259; (2) the publication year ranges from 1997 to 2019; (3) 52 studies performed immunohistochemical (IHC) staining to evaluate NK cell markers, while one study conducted flow cytometry (FCM) analysis, and three studies conducted real-time polymerase chain reaction (RT-PCR); (4) HRs and 95%CIs were not directly obtained from eighteen included studies; and (5) the NOS score ranges from 6 to 8.
Prognostic Value of NK Cell Marker CD56 in Patients With Solid Tumors
Pooled results of 18 studies comprising 2,882 patients revealed that CD56 was significantly correlated with better OS (HR = 0.473, 95%CI: 0.315–0.710, p < 0.001) (Figure 2A; Table 2). The random-effect model was more appropriate owing to the obvious heterogeneity among the included studies (Cochrane Q, p < 0.001; I2 = 70.0%). In the sensitivity analysis, the elimination of any cohort failed to change the statistical significance, which further confirmed the stability and credibility of pooled results (Figure 2B). Begg's test indicated significant publication bias in the analysis of the association between CD56 and OS (p = 0.049) (Figure 2C). Moreover, pooled HR of 5 pieces of research which performed Cox multivariate analysis showed that a high level of CD56 served as an independent prognostic predictor for favorable OS of solid tumor patients (HR = 0.372, 95%CI: 0.261–0.531, p < 0.001) (Figure 2D; Table 2).
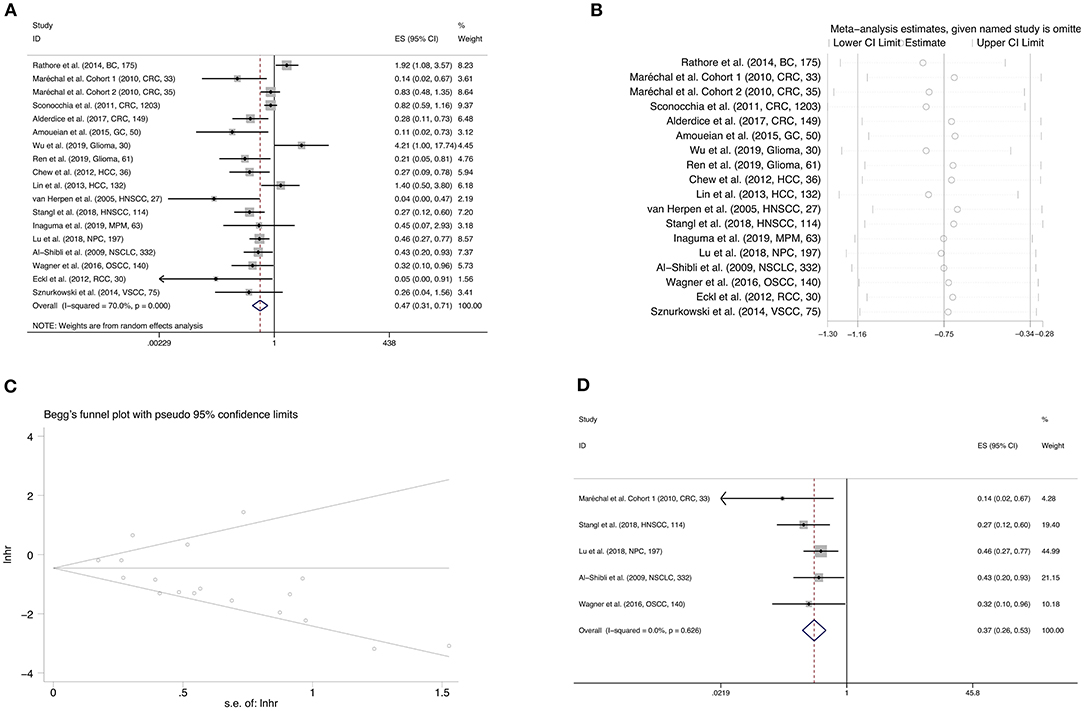
Figure 2. Forest plot (A), sensitivity analysis plot (B), and Begg's test (C) of the meta-analysis of OS for solid tumor patients divided by the level of CD56. Forest plot (D) of the meta-analysis of OS for solid tumor patients divided by the level of CD56, from Cox multivariate analysis. In the forest plots, each study ID was set as the following format: authors (year, tumor type, sample size).
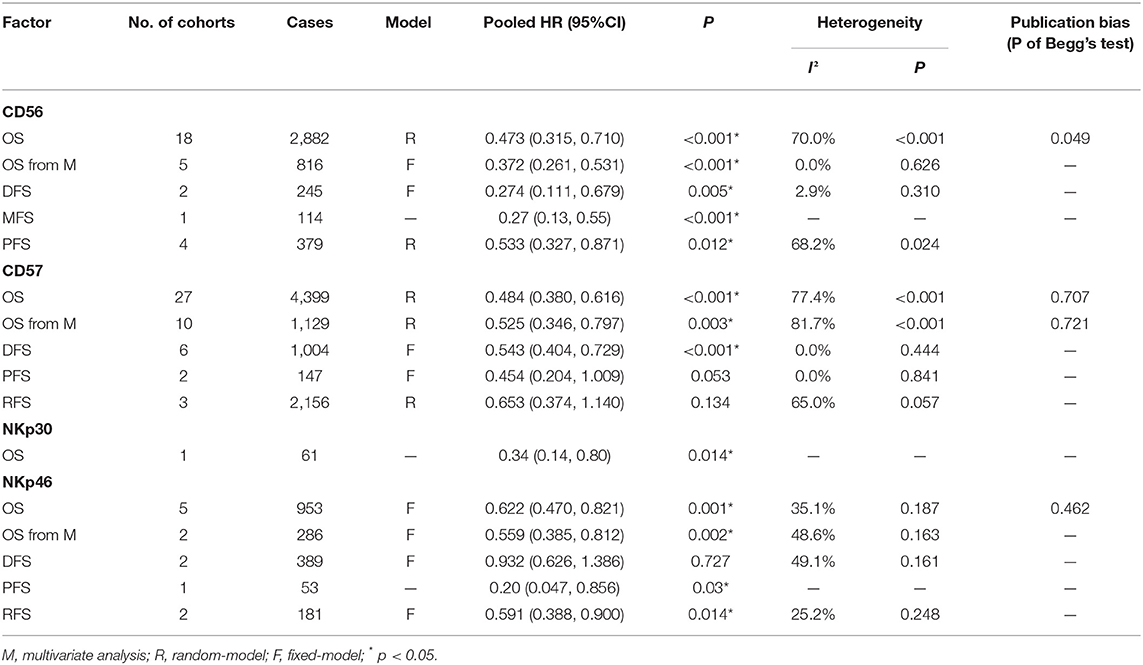
Table 2. Pooled HR, heterogeneity, and publication bias of the meta-analysis of OS, DFS, MFS, PFS, and RFS in patients with solid tumors.
Furthermore, we assessed the prognostic significance of CD56 in certain types of tumors. The results showed that a high level of CD56 predicted better OS in head and neck squamous cell carcinoma (HNSCC) (HR = 0.356, 95%CI: 0.237–0.533, p < 0.001) (Supplementary Figure 1A), but not in colorectal cancer (CRC) (HR = 0.574, 95%CI: 0.328–1.004, p = 0.052) (Supplementary Figure 1B), glioma (HR = 0.933, 95%CI: 0.050–17.523, p = 0.963) (Supplementary Figure 1C), or HCC (HR = 0.620, 95%CI: 0.124–3.109, p = 0.561) (Supplementary Figure 1D; Table 3).
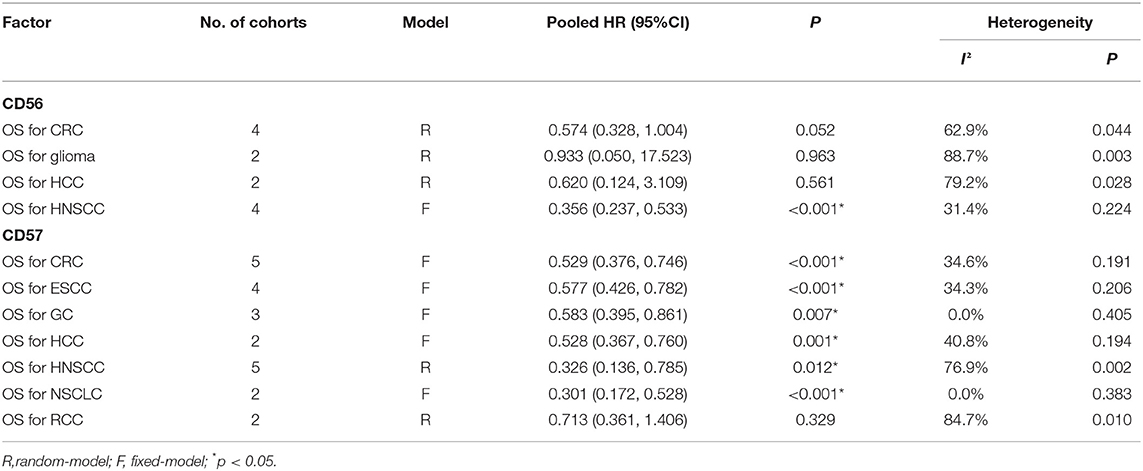
Table 3. Pooled HR and heterogeneity of the meta-analysis of OS in patients with certain types of solid tumors.
The impact of CD56 on the prognosis of solid malignancies was investigated in 2 studies comprising 245 cases for DFS and 4 studies comprising 379 cases for PFS. A fixed-effect model was appropriate for the analysis of DFS (Cochrane Q, p = 0.310; I2 = 2.9%), while random effect model was used for PFS due to obvious heterogeneity (Cochrane Q, p = 0.024; I2 = 68.2%). The results displayed that CD56 was significantly correlated with favorable DFS (HR = 0.274, 95%CI: 0.111–0.679, p = 0.005) (Supplementary Figure 1E) and favorable PFS (HR = 0.533, 95%CI: 0.327–0.871, p = 0.012) (Supplementary Figure 1F) in patients with solid tumors (Table 2).
Prognostic Value of NK Cell Marker CD57 in Patients With Solid Tumors
To estimate the prognostic significance of CD57 in OS, we pooled the data from 27 studies involving 4,399 patients. A random effect model was more appropriate in the analysis of OS due to the obvious inter-study heterogeneity (Cochrane Q, p < 0.001; I2 = 77.4%). Our results revealed CD57 to be significantly correlated with favorable OS in patients with solid tumors (HR = 0.484, 95%CI: 0.380–0.616, p < 0.001) (Figure 3A; Table 2). The original statistical significance was not altered after omitting any cohorts in the sensitivity analysis (Figure 3B), and publication bias was not detected by Begg's test (p = 0.707) (Figure 3C). In addition, pooled HR of 10 pieces of research conducting Cox multivariate analysis showed that a high level of CD57 in solid tumors could independently predict better OS (HR = 0.525, 95%CI: 0.346–0.797, p = 0.003) (Figure 3D; Table 2).
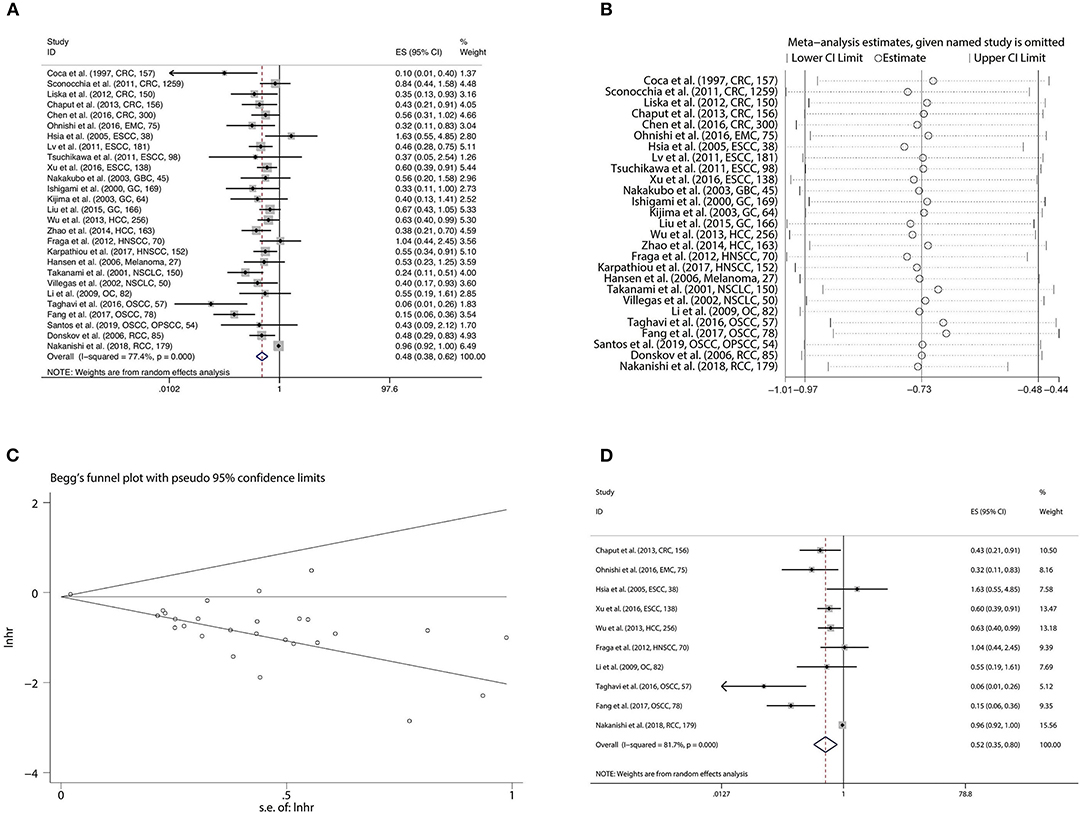
Figure 3. Forest plot (A), sensitivity analysis plot (B), and Begg's test (C) of the meta-analysis of OS for solid tumor patients divided by the level of CD57. Forest plot (D) of the meta-analysis of OS for solid tumor patients divided by the level of CD57, from Cox multivariate analysis.
The prognostic significance of CD57 in certain types of tumors was further assessed. CD57 predicted improved OS in colorectal cancer (CRC) (HR = 0.529, 95%CI: 0.376–0.746, p < 0.001) (Supplementary Figure 2A), esophageal squamous cell carcinoma (ESCC) (HR = 0.577, 95%CI: 0.426–0.782, p < 0.001) (Supplementary Figure 2B), gastric cancer (GC) (HR = 0.583, 95%CI: 0.395–0.861, p = 0.007) (Supplementary Figure 2C), HCC (HR = 0.528, 95%CI: 0.367–0.760, p = 0.001) (Supplementary Figure 2D), HNSCC (HR = 0.326, 95%CI: 0.136–0.785, p = 0.012) (Supplementary Figure 2E), and non-small cell lung cancer (NSCLC) (HR = 0.301, 95%CI: 0.172–0.528, p < 0.001) (Supplementary Figure 2F), but not in renal cell carcinoma (RCC) (HR = 0.713, 95%CI: 0.361–1.406, p = 0.329) (Supplementary Figure 2G; Table 3).
Furthermore, the impact of CD57 on the prognosis of solid malignancies was investigated in 6 studies comprising 1,004 cases for DFS, 2 studies comprising 147 cases for PFS, and 3 studies comprising 2,156 cases for RFS. A fixed-effect model was preferred in the analyses of DFS (Cochrane Q, p = 0.444; I2 = 0.0%) and PFS (Cochrane Q, p = 0.841; I2 = 0.0%), while random effect model was used for RFS owing to obvious heterogeneity (Cochrane Q, p = 0.057; I2 = 65.0%). The results displayed that CD57 was significantly associated with better DFS in patients with solid malignancies (HR = 0.543, 95%CI: 0.404–0.729, p < 0.001) (Supplementary Figure 2H). However, the prognostic significance of CD57 was not detected in the analysis of PFS (HR = 0.454, 95%CI: 0.204–1.009, p = 0.053) (Supplementary Figure 2I) or RFS (HR = 0.653, 95%CI: 0.374–1.140, p = 0.134) (Supplementary Figure 2J; Table 2).
Prognostic Value of NK Markers NKp30 and NKp46 in Patients With Solid Tumors
Only one piece of original research (77) conducting RT-PCR of NKp30 was included in the current study, which showed that a high level of NKp30 could predict better OS of HCC patients (HR = 0.34, 95%CI: 0.14–0.80, p = 0.014) (Table 2). Thus, the prognostic value of NKp30 is inconclusive in the current meta-analysis due to limited eligible studies.
The prognostic value of NKp46 was explored in 7 observation studies, including 5 for OS, 2 for DFS, 1 for PFS, and 2 for RFS. NKp46 was significantly correlated with favorable OS (HR = 0.622, 95%CI: 0.470–0.821, p = 0.001) (Figure 4A) and RFS (HR = 0.591, 95%CI: 0.388–0.900, p = 0.014). However, statistical significance of NKp46 in DFS was not detected by the present meta-analysis (HR = 0.932, 95%CI: 0.626–1.386, p = 0.727) (Table 2). In addition, pooled HR of two Cox multivariate analyses demonstrated that NKp46 was an independent prognostic predictor of better OS (HR = 0.559, 95%CI: 0.385–0.812, p = 0.002) (Figure 4B; Table 2).
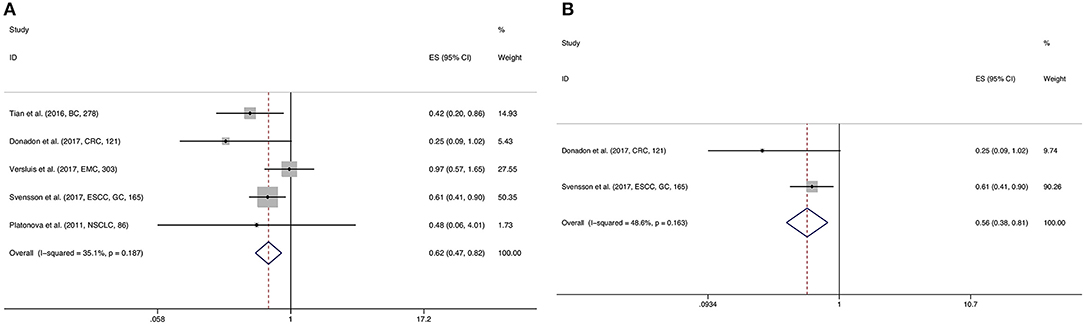
Figure 4. Forest plot (A) of the meta-analysis of OS for solid tumor patients divided by the level of NKp46. Forest plot (B) of the meta-analysis of OS for solid tumor patients divided by the level of NKp46, from Cox multivariate analysis.
Discussion
TINKs play a significant role in tumor defense, which might be related to tumor prognosis. However, inconsistent results were reported by previous clinical studies and some studies suggested that TINKs are not capable of efficient tumor lysis (85, 86). The present meta-analysis comprehensively analyzed the prognostic significance of NK cell markers in solid tumors. We analyzed the results of 56 independent original studies and detected that high levels of NK cell markers CD56, CD57, NKp30, or NKp46 were all correlated with better prognosis of solid tumor patients.
NK cells were defined as CD3−CD56+ lymphocytes. Based on the density of CD56, NK cells are divided into CD56bright and CD56dim subsets. CD56 could drive the maturation of NK cells and act as a reliable marker for TINKs (87). Normally, more than 90% of PBNKs are CD16+CD56dim NK cells, which are a more matured and more cytotoxic subset, while CD56bright NK cells are a less matured subset that comprise 5–10% of PBNKs (11, 88). CD56bright NK cells were considered as strong “cytokine producers” that secrete anti-tumor cytokines such as IFN-γ in response to IL-2, IL-12, and IL-18 (24). Carrega et al. also indicated that NK cells infiltrating NSCLC mainly exerted cytokine producing effects rather than direct tumor cell killing effects (89). Correspondingly, the current meta-analysis indicated that a high level of CD56 could predict improved OS, DFS, and PFS in solid tumor patients. In addition, our results indicated that CD56 could serve as an independent predictor of improved OS. Nevertheless, discrepant results were reached in the analysis of certain tumor types, which might be attributed to the limited number of eligible studies.
CD57 is another crucial surface marker of NK cells, which represents the terminal maturation of NK cells (22, 90). CD57+ NK cells are featured with less proliferation and less IFN-γ production, but with higher cytotoxicity to tumor cells in comparison with CD57− NK cells (25). Previous studies also reported that CD57+ NK cells could reacquire the IFN-γ producing potential when crosslinked with CD16 (22, 91). Therefore, appropriate proportion of CD57+ and CD57− NK cells could have synergistic anti-tumor effects, and therefore benefit the survival of tumor-bearing patients (92, 93). After stimulation by tumor-associated antigens, CD57+ NK cells are accumulated in various solid tumor tissues (48–50, 94). Thus, a great deal of research attempted to clarify the clinical role of a high level of CD57+ TINKs, which might be a signal for effective tumor defense. We analyzed the data from 31 included studies and the results suggested that a high level of CD57 was correlated with improved OS and DFS in patients with solid tumors in the current meta-analysis. Moreover, high level of CD57 predicted improved OS in CRC, ESCC, GC, HCC, HNSCC, and NSCLC in the analysis of certain types of tumors.
In recent decades, three natural cytotoxicity receptor (NCR) members of NK cells have been identified, including NKp30 (NCR3), NKp44 (NCR2), and NKp46 (NCR1). Six different splice variants of NKp30 (NKp30a-e) were expressed on the cell surface and each of them has a specific function (95). NKp30a and NKp30b were found to induce cytotoxicity and cytokine production respectively, whereas NKp30c showed an immunosuppressive activity in previous research (96). Only one study on NKp30 was included in this meta-analysis, which reported that a high level of NKp30 was associated with improved OS of HCC (77). Therefore, its prognostic value needs to be further explored. In terms of NKp46, Halfteck et al. found that tumor growth was enhanced in NKp46-deficient mice, which highlighted the vital role of NKp46+ NK cells in anti-tumor defense (97). Moreover, NKp46 could upregulate tumor necrosis factor-related apoptosis-inducing ligand (TRAIL), thus enhancing the anti-tumor effects of NK cells (98). Currently, our results indicated that a high level of NKp46 was significantly associated with favorable OS and RFS.
Our results imply some concerns regarding current clinical research. On the basis of TNM staging system, a more accurate prognosis prediction system according to the level of these markers could be built in the future, to direct the selection of therapeutic regimens. Further studies are highly encouraged to explore the regulatory mechanisms of the tumor-infiltrating processes of NK cells and the enhancement methods of the anti-tumor effects of TINKs (99). In addition, targeting the function state of NK cells might be a promising treatment strategy for solid tumors, such as IL-15-mediated CD56 activation (100). However, great care is needed when extrapolating the prognostic value into treatment potential.
Notably, discrepant results were achieved in the current study, which might decrease the evidence grade to some extent. The inconsistent results might be attributed to a limited number of included studies in a certain analysis. In addition, obvious inter-study heterogeneity existed in the analysis of the prognostic significance of CD56 and CD57 in OS. We speculated that the following factors might be the sources of heterogeneity: (1) Tumor types and clinical stages differed among included studies, (2) Detection and cut-off methodologies differed, and (3) Some included studies were featured with a small sample size or short follow-up period.
Finally, several limitations of this work should be underlined. Firstly, the detection and cut-off methodologies were discrepant among included studies, although IHC staining and scoring of the number of positive stained cells were conducted by most studies. Secondly, the data were not directly obtained from 18 included articles. The processes of calculating HRs and 95% CIs by survival curves might result in data inaccuracy to some extent. Thirdly, different pathological patterns, pathological grades, clinical stages, and therapeutic regimens among the included studies limited the credibility and applicability of the conclusions. Fourthly, although most included studies detected NK cell markers to identify the degree of NK cell tumor-infiltration, it should be stated that the prognostic value of NK cell markers might be attributed to the synthetic effects of multiple immune cells for the reason that other types of immune cells, such as NK-T cells, dendritic cells, and monocytes, partially shared some surface markers of NK cells (101). Fifthly, the potential publication bias in the current analysis should not be overlooked, which might be derived from the missing of non-English articles or unpublished results.
Conclusions
Our results suggested that high levels of NK cell markers CD56, CD57, NKp30, and NKp46 were significantly correlated with favorable outcomes for solid tumor patients, providing theoretical evidence for further accurate prognosis prediction of solid tumors. However, to transfer the conclusions into a clinical application, a specific scoring system should be established and tested with prospective and high-quality researches.
Data Availability Statement
The datasets analyzed for this study are available from the corresponding author (ZS).
Author Contributions
ZS conceived of and designed the study. SZ and WL collected, extracted, and analyzed the data. BH, PW, XL, and SC performed the quality assessment and analyzed the data. SZ wrote the paper. All authors read and approved the manuscript.
Funding
This study was supported by Grants 2016YFC1100100 from The National Key Research and Development Program of China, Grants 91649204 from Major Research Plan of National Natural Science Foundation of China.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We would like to thank the researchers for their contributions.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2020.01242/full#supplementary-material
Abbreviations
TINK, tumor-infiltrating natural killer cell; HR, hazard ratio; CI, confidence interval; IFN, interferon; NCR, natural cytotoxicity receptor; IHC, immunohistochemical; FCM, flow cytometry; RT-PCR, real-time polymerase chain reaction; OS, overall survival; DFS, disease-free survival; MFS, metastasis-free survival; PFS, progression-free survival; RFS, recurrence-free survival; NOS, Newcastle-Ottawa Scale.
References
1. Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. (2015) 136:E359–86. doi: 10.1002/ijc.29210
2. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. (2020) 70:7–30. doi: 10.3322/caac.21590
3. Torre LA, Siegel RL, Ward EM, Jemal A. Global cancer incidence and mortality rates and trends–an update. Cancer Epidemiol Biomarkers Prev. (2016) 25:16–27. doi: 10.1158/1055-9965.Epi-15-0578
4. Cortes J, Perez-García JM, Llombart-Cussac A, Curigliano G, El Saghir NS, Cardoso F, et al. Enhancing global access to cancer medicines. CA Cancer J Clin. (2020) 70:105–24. doi: 10.3322/caac.21597
5. Sasada T, Suekane S. Variation of tumor-infiltrating lymphocytes in human cancers: controversy on clinical significance. Immunotherapy. (2011) 3:1235–51. doi: 10.2217/imt.11.106
6. López-Soto A, Gonzalez S, Smyth MJ, Galluzzi L. Control of metastasis by NK cells. Cancer Cell. (2017) 32:135–54. doi: 10.1016/j.ccell.2017.06.009
7. Malmberg KJ, Carlsten M, Bjorklund A, Sohlberg E, Bryceson YT, Ljunggren HG. Natural killer cell-mediated immunosurveillance of human cancer. Semin Immunol. (2017) 31:20–9. doi: 10.1016/j.smim.2017.08.002
8. Imai K, Matsuyama S, Miyake S, Suga K, Nakachi K. Natural cytotoxic activity of peripheral-blood lymphocytes and cancer incidence: an 11-year follow-up study of a general population. Lancet. (2000) 356:1795–9. doi: 10.1016/s0140-6736(00)03231-1
9. Ruggeri L, Capanni M, Urbani E, Perruccio K, Shlomchik WD, Tosti A, et al. Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science. (2002) 295(5562):2097–100. doi: 10.1126/science.1068440
10. Ljunggren HG, Malmberg KJ. Prospects for the use of NK cells in immunotherapy of human cancer. Nat Rev Immunol. (2007) 7:329–39. doi: 10.1038/nri2073
11. Vivier E, Tomasello E, Baratin M, Walzer T, Ugolini S. Functions of natural killer cells. Nat Immunol. (2008) 9:503–10. doi: 10.1038/ni1582
12. Zhu Y, Huang B, Shi J. Fas ligand and lytic granule differentially control cytotoxic dynamics of natural killer cell against cancer target. Oncotarget. (2016) 7:47163–72. doi: 10.18632/oncotarget.9980
13. Smyth MJ, Thia KY, Cretney E, Kelly JM, Snook MB, Forbes CA, et al. Perforin is a major contributor to NK cell control of tumor metastasis. J Immunol. (1999) 162:6658–62.
14. Bassani B, Baci D, Gallazzi M, Poggi A, Bruno A, Mortara L. Natural killer cells as key players of tumor progression and angiogenesis: old and novel tools to divert their pro-tumor activities into potent anti-tumor effects. Cancers. (2019) 11:461. doi: 10.3390/cancers11040461
15. Clayton A, Mitchell JP, Court J, Linnane S, Mason MD, Tabi Z. Human tumor-derived exosomes down-modulate NKG2D expression. J Immunol. (2008) 180:7249. doi: 10.4049/jimmunol.180.11.7249
16. Coudert JD, Scarpellino L, Gros F, Vivier E, Held W. Sustained NKG2D engagement induces cross-tolerance of multiple distinct NK cell activation pathways. Blood. (2008) 111:3571–8. doi: 10.1182/blood-2007-07-100057
17. Yang C, Cheng H, Zhang Y, Fan K, Luo G, Fan Z, et al. Anergic natural killer cells educated by tumor cells are associated with a poor prognosis in patients with advanced pancreatic ductal adenocarcinoma. Cancer Immunol Immunother. (2018) 67:1815–23. doi: 10.1007/s00262-018-2235-8
18. Sun H, Huang Q, Huang M, Wen H, Lin R, Zheng M, et al. Human CD96 correlates to natural killer cell exhaustion and predicts the prognosis of human hepatocellular carcinoma. Hepatology. (2019) 70:168–83. doi: 10.1002/hep.30347
19. Bruno A, Ferlazzo G, Albini A, Noonan DM. A think tank of TINK/TANKs: tumor-infiltrating/tumor-associated natural killer cells in tumor progression and angiogenesis. J Natl Cancer Inst. (2014) 106:dju200. doi: 10.1093/jnci/dju200
20. Bruno A, Focaccetti C, Pagani A, Imperatori AS, Spagnoletti M, Rotolo N, et al. The proangiogenic phenotype of natural killer cells in patients with non-small cell lung cancer. Neoplasia. (2013) 15:133–42. doi: 10.1593/neo.121758
21. Horowitz A, Strauss-Albee DM, Leipold M, Kubo J, Nemat-Gorgani N, Dogan OC, et al. Genetic and environmental determinants of human NK cell diversity revealed by mass cytometry. Sci Transl Med. (2013) 5:208ra145. doi: 10.1126/scitranslmed.3006702
22. Björkström NK, Riese P, Heuts F, Andersson S, Fauriat C, Ivarsson MA, et al. Expression patterns of NKG2A, KIR, and CD57 define a process of CD56dim NK-cell differentiation uncoupled from NK-cell education. Blood. (2010) 116:3853–64. doi: 10.1182/blood-2010-04-281675
23. Fu B, Tian Z, Wei H. Subsets of human natural killer cells and their regulatory effects. Immunology. (2014) 141:483–9. doi: 10.1111/imm.12224
24. Cooper MA, Fehniger TA, Caligiuri MA. The biology of human natural killer-cell subsets. Trends Immunol. (2001) 22:633–40. doi: 10.1016/s1471-4906(01)02060-9
25. Nielsen CM, White MJ, Goodier MR, Riley EM. Functional significance of CD57 expression on human NK cells and relevance to disease. Front Immunol. (2013) 4:422. doi: 10.3389/fimmu.2013.00422
26. Vieillard V, Baychelier F, Debre P. NKp44L: a new tool for fighting cancer. Oncoimmunology. (2014) 3:e27988. doi: 10.4161/onci.27988
27. Amoueian S, Attaranzadeh A, Montazer M. Intratumoral CD68-, CD117-, CD56-, and CD1a-positive immune cells and the survival of Iranian patients with non-metastatic intestinal-type gastric carcinoma. Pathol Res Pract. (2015) 211:326–31. doi: 10.1016/j.prp.2014.12.013
28. Mandal R, Senbabaoglu Y, Desrichard A, Havel JJ, Dalin MG, Riaz N, et al. The head and neck cancer immune landscape and its immunotherapeutic implications. JCI Insight. (2016) 1:e89829. doi: 10.1172/jci.insight.89829
29. Hsia JY, Chen JT, Chen CY, Hsu CP, Miaw J, Huang YS, et al. Prognostic significance of intratumoral natural killer cells in primary resected esophageal squamous cell carcinoma. Chang Gung Med J. (2005) 28:335–40.
30. Rathore AS, Goel MM, Makker A, Kumar S, Srivastava AN. Is the tumor infiltrating natural killer cell (NK-TILs) count in infiltrating ductal carcinoma of breast prognostically significant? Asian Pac J Cancer Prev. (2014) 15:3757–61. doi: 10.7314/apjcp.2014.15.8.3757
31. Stanley TD, Doucouliagos H. Meta-regression approximations to reduce publication selection bias. Res Synth Methods. (2014) 5:60–78. doi: 10.1002/jrsm.1095
32. van Herpen CM, van der Laak JA, de Vries IJ, van Krieken JH, de Wilde PC, Balvers MG, et al. Intratumoral recombinant human interleukin-12 administration in head and neck squamous cell carcinoma patients modifies locoregional lymph node architecture and induces natural killer cell infiltration in the primary tumor. Clin Cancer Res. (2005) 11:1899–909. doi: 10.1158/1078-0432.ccr-04-1524
33. Al-Shibli K, Al-Saad S, Donnem T, Persson M, Bremnes RM, Busund LT. The prognostic value of intraepithelial and stromal innate immune system cells in non-small cell lung carcinoma. Histopathology. (2009) 55:301–12. doi: 10.1111/j.1365-2559.2009.03379.x
34. Marechal R, De Schutter J, Nagy N, Demetter P, Lemmers A, Deviere J, et al. Putative contribution of CD56 positive cells in cetuximab treatment efficacy in first-line metastatic colorectal cancer patients. BMC Cancer. (2010) 10:340. doi: 10.1186/1471-2407-10-340
35. Sconocchia G, Zlobec I, Lugli A, Calabrese D, Iezzi G, Karamitopoulou E, et al. Tumor infiltration by FcgammaRIII (CD16)+ myeloid cells is associated with improved survival in patients with colorectal carcinoma. Int J Cancer. (2011) 128:2663–72. doi: 10.1002/ijc.25609
36. Eckl J, Buchner A, Prinz PU, Riesenberg R, Siegert SI, Kammerer R, et al. Transcript signature predicts tissue NK cell content and defines renal cell carcinoma subgroups independent of TNM staging. J Mol Med. (2012) 90:55–66. doi: 10.1007/s00109-011-0806-7
37. Chew V, Chen J, Lee D, Loh E, Lee J, Lim KH, et al. Chemokine-driven lymphocyte infiltration: an early intratumoural event determining long-term survival in resectable hepatocellular carcinoma. Gut. (2012) 61:427–38. doi: 10.1136/gutjnl-2011-300509
38. Lin SZ, Chen KJ, Xu ZY, Chen H, Zhou L, Xie HY, et al. Prediction of recurrence and survival in hepatocellular carcinoma based on two Cox models mainly determined by FoxP3+ regulatory T cells. Cancer Prev Res. (2013) 6:594–602. doi: 10.1158/1940-6207.capr-12-0379
39. Sznurkowski JJ, Zawrocki A, Biernat W. Subtypes of cytotoxic lymphocytes and natural killer cells infiltrating cancer nests correlate with prognosis in patients with vulvar squamous cell carcinoma. Cancer Immunol Immunother. (2014) 63:297–303. doi: 10.1007/s00262-013-1511-x
40. Wagner S, Wittekindt C, Reuschenbach M, Hennig B, Thevarajah M, Wurdemann N, et al. CD56-positive lymphocyte infiltration in relation to human papillomavirus association and prognostic significance in oropharyngeal squamous cell carcinoma. Int J Cancer. (2016) 138:2263–73. doi: 10.1002/ijc.29962
41. Alderdice M, Dunne PD, Cole AJ, O'Reilly PG, McArt DG, Bingham V, et al. Natural killer-like signature observed post therapy in locally advanced rectal cancer is a determinant of pathological response and improved survival. Mod Pathol. (2017) 30:1287–98. doi: 10.1038/modpathol.2017.47
42. Lu J, Chen X-M, Huang H-R, Zhao F-P, Wang F, Liu X, et al. Detailed analysis of inflammatory cell infiltration and the prognostic impact on nasopharyngeal carcinoma. Head Neck. (2018) 40:1245–53. doi: 10.1002/hed.25104
43. Stangl S, Tontcheva N, Sievert W, Shevtsov M, Niu M, Schmid TE, et al. Heat shock protein 70 and tumor-infiltrating NK cells as prognostic indicators for patients with squamous cell carcinoma of the head and neck after radiochemotherapy: a multicentre retrospective study of the German Cancer Consortium Radiation Oncology Group (DKTK-ROG). Int J Cancer. (2018) 142:1911–25. doi: 10.1002/ijc.31213
44. Inaguma S, Lasota J, Czapiewski P, Langfort R, Rys J, Szpor J, et al. CD70 expression correlates with a worse prognosis in malignant pleural mesothelioma patients via immune evasion and enhanced invasiveness. J Pathol. (2019) 250:205–16. doi: 10.1002/path.5361
45. Muntasell A, Rojo F, Servitja S, Rubio-Perez C, Cabo M, Tamborero D, et al. NK cell infiltrates and HLA Class I expression in primary HER2(+) breast cancer predict and uncouple pathological response and disease-free survival. Clin Cancer Res. (2019) 25:1535–45. doi: 10.1158/1078-0432.ccr-18-2365
46. Ren F, Zhao Q, Huang L, Zheng Y, Li L, He Q, et al. The R132H mutation in IDH1 promotes the recruitment of NK cells through CX3CL1/CX3CR1 chemotaxis and is correlated with a better prognosis in gliomas. Immunol Cell Biol. (2019) 97:457–69. doi: 10.1111/imcb.12225
47. Wu S, Yang W, Zhang H, Ren Y, Fang Z, Yuan C, et al. The prognostic landscape of tumor-infiltrating immune cells and immune checkpoints in glioblastoma. Technol Cancer Res Treat. (2019) 18:1533033819869949. doi: 10.1177/1533033819869949
48. Coca S, PerezPiqueras J, Martinez D, Colmenarejo A, Saez MA, Vallejo C, et al. The prognostic significance of intratumoral natural killer cells in patients with colorectal carcinoma. Cancer. (1997) 79:2320–8. doi: 10.1002/(sici)1097-0142(19970615)79:12<2320::aid-cncr5>3.0.co;2-p
49. Ishigami S, Natsugoe S, Tokuda K, Nakajo A, Xiangming C, Iwashige H, et al. Clinical impact of intratumoral natural killer cell and dendritic cell infiltration in gastric cancer. Cancer Lett. (2000) 159:103–8. doi: 10.1016/s0304-3835(00)00542-5
50. Takanami I, Takeuchi K, Giga M. The prognostic value of natural killer cell infiltration in resected pulmonary adenocarcinoma. J Thorac Cardiovasc Surg. (2001) 121:1058–63. doi: 10.1067/mtc.2001.113026
51. Villegas FR, Coca S, Villarrubia VG, Jimenez R, Chillon MJ, Jareno J, et al. Prognostic significance of tumor infiltrating natural killer cells subset CD57 in patients with squamous cell lung cancer. Lung Cancer. (2002) 35:23–8. doi: 10.1016/s0169-5002(01)00292-6
52. Kijima Y, Ishigami S, Hokita S, Koriyama C, Akiba S, Eizuru Y, et al. The comparison of the prognosis between Epstein-Barr virus (EBV)-positive gastric carcinomas and EBV-negative ones. Cancer Lett. (2003) 200:33–40. doi: 10.1016/s0304-3835(03)00410-5
53. Nakakubo Y, Miyamoto M, Cho Y, Hida Y, Oshikiri T, Suzuoki M, et al. Clinical significance of immune cell infiltration within gallbladder cancer. Br J Cancer. (2003) 89:1736–42. doi: 10.1038/sj.bjc.6601331
54. Menon AG, Janssen-van Rhijn CM, Morreau H, Putter H, Tollenaar RA, van de Velde CJ, et al. Immune system and prognosis in colorectal cancer: a detailed immunohistochemical analysis. Lab Invest. (2004) 84:493–501. doi: 10.1038/labinvest.3700055
55. Donskov F, von der Maase H. Impact of immune parameters on long-term survival in metastatic renal cell carcinoma. J Clin Oncol. (2006) 24:1997–2005. doi: 10.1200/jco.2005.03.9594
56. Hansen BD, Schmidt H, von der Maase H, Sjoegren P, Agger R, Hokland M. Tumour-associated macrophages are related to progression in patients with metastatic melanoma following interleukin-2 based immunotherapy. Acta Oncol. (2006) 45:400–5. doi: 10.1080/02841860500471798
57. Ino K, Yamamoto E, Shibata K, Kajiyama H, Yoshida N, Terauchi M, et al. Inverse correlation between tumoral indoleamine 2,3-dioxygenase expression and tumor-infiltrating lymphocytes in endometrial cancer: its association with disease progression and survival. Clin Cancer Res. (2008) 14:2310–7. doi: 10.1158/1078-0432.ccr-07-4144
58. Li K, Mandai M, Hamanishi J, Matsumura N, Suzuki A, Yagi H, et al. Clinical significance of the NKG2D ligands, MICA/B and ULBP2 in ovarian cancer: high expression of ULBP2 is an indicator of poor prognosis. Cancer Immunol Immunother. (2009) 58:641–52. doi: 10.1007/s00262-008-0585-3
59. Lv L, Pan K, Li XD, She KL, Zhao JJ, Wang W, et al. The accumulation and prognosis value of tumor infiltrating IL-17 producing cells in esophageal squamous cell carcinoma. PLoS ONE. (2011) 6:e18219. doi: 10.1371/journal.pone.0018219
60. Tsuchikawa T, Ikeda H, Cho Y, Miyamoto M, Shichinohe T, Hirano S, et al. Association of CD8+ T cell infiltration in oesophageal carcinoma lesions with human leucocyte antigen (HLA) class I antigen expression and survival. Clin Exp Immunol. (2011) 164:50–6. doi: 10.1111/j.1365-2249.2010.04311.x
61. Fraga CA, de Oliveira MV, Domingos PL, Botelho AC, Guimaraes AL, Teixeira-Carvalho A, et al. Infiltrating CD57+ inflammatory cells in head and neck squamous cell carcinoma: clinicopathological analysis and prognostic significance. Appl Immunohistochem Mol Morphol. (2012) 20:285–90. doi: 10.1097/PAI.0b013e318228357b
62. Liska V, Vycital O, Daum O, Novak P, Treska V, Bruha J, et al. Infiltration of colorectal carcinoma by S100+ dendritic cells and CD57+ lymphocytes as independent prognostic factors after radical surgical treatment. Anticancer Res. (2012) 32:2129–32.
63. Chaput N, Svrcek M, Auperin A, Locher C, Drusch F, Malka D, et al. Tumour-infiltrating CD68+ and CD57+ cells predict patient outcome in stage II-III colorectal cancer. Br J Cancer. (2013) 109:1013–22. doi: 10.1038/bjc.2013.362
64. Wu Y, Kuang DM, Pan WD, Wan YL, Lao XM, Wang D, et al. Monocyte/macrophage-elicited natural killer cell dysfunction in hepatocellular carcinoma is mediated by CD48/2B4 interactions. Hepatology. (2013) 57:1107–16. doi: 10.1002/hep.26192
65. Wangerin H, Kristiansen G, Schlomm T, Stephan C, Gunia S, Zimpfer A, et al. CD57 expression in incidental, clinically manifest, and metastatic carcinoma of the prostate. Biomed Res Int. (2014) 2014:356427. doi: 10.1155/2014/356427
66. Zhao JJ, Pan QZ, Pan K, Weng DS, Wang QJ, Li JJ, et al. Interleukin-37 mediates the antitumor activity in hepatocellular carcinoma: role for CD57+ NK cells. Sci Rep. (2014) 4:5177. doi: 10.1038/srep05177
67. Hernandez-Prieto S, Romera A, Ferrer M, Subiza JL, Lopez-Asenjo JA, Jarabo JR, et al. A 50-gene signature is a novel scoring system for tumor-infiltrating immune cells with strong correlation with clinical outcome of stage I/II non-small cell lung cancer. Clin Transl Oncol. (2015) 17:330–8. doi: 10.1007/s12094-014-1235-1
68. Liu K, Yang K, Wu B, Chen H, Chen X, Chen X, et al. Tumor-infiltrating immune cells are associated with prognosis of gastric cancer. Medicine. (2015) 94:e1631. doi: 10.1097/md.0000000000001631
69. Chen Y, Yuan R, Wu X, He X, Zeng Y, Fan X, et al. A novel immune marker model predicts oncological outcomes of patients with colorectal cancer. Ann Surg Oncol. (2016) 23:826–32. doi: 10.1245/s10434-015-4889-1
70. Ohnishi K, Yamaguchi M, Erdenebaatar C, Saito F, Tashiro H, Katabuchi H, et al. Prognostic significance of CD169-positive lymph node sinus macrophages in patients with endometrial carcinoma. Cancer Sci. (2016) 107:846–52. doi: 10.1111/cas.12929
71. Taghavi N, Bagheri S, Akbarzadeh A. Prognostic implication of CD57, CD16, and TGF-beta expression in oral squamous cell carcinoma. J Oral Pathol Med. (2016) 45:58–62. doi: 10.1111/jop.12320
72. Xu B, Chen L, Li J, Zheng X, Shi L, Wu C, et al. Prognostic value of tumor infiltrating NK cells and macrophages in stage II+III esophageal cancer patients. Oncotarget. (2016) 7:74904–16. doi: 10.18632/oncotarget.12484
73. Fang J, Li X, Ma D, Liu X, Chen Y, Wang Y, et al. Prognostic significance of tumor infiltrating immune cells in oral squamous cell carcinoma. BMC Cancer. (2017) 17:375. doi: 10.1186/s12885-017-3317-2
74. Karpathiou G, Casteillo F, Giroult JB, Forest F, Fournel P, Monaya A, et al. Prognostic impact of immune microenvironment in laryngeal and pharyngeal squamous cell carcinoma: immune cell subtypes, immuno-suppressive pathways and clinicopathologic characteristics. Oncotarget. (2017) 8:19310–22. doi: 10.18632/oncotarget.14242
75. Nakanishi H, Miyata Y, Mochizuki Y, Yasuda T, Nakamura Y, Araki K, et al. Pathological significance and prognostic roles of densities of CD57+ cells, CD68+ cells, and mast cells, and their ratios in clear cell renal cell carcinoma. Hum Pathol. (2018) 79:102–8. doi: 10.1016/j.humpath.2018.05.007
76. Santos EM, Rodrigues de Matos F, Freitas de Morais E, Galvao HC, de Almeida Freitas R. Evaluation of Cd8+ and natural killer cells defense in oral and oropharyngeal squamous cell carcinoma. J Craniomaxillofac Surg. (2019) 47:676–81. doi: 10.1016/j.jcms.2019.01.036
77. Chew V, Tow C, Teo M, Wong HL, Chan J, Gehring A, et al. Inflammatory tumour microenvironment is associated with superior survival in hepatocellular carcinoma patients. J Hepatol. (2010) 52:370–9. doi: 10.1016/j.jhep.2009.07.013
78. Platonova S, Cherfils-Vicini J, Damotte D, Crozet L, Vieillard V, Validire P, et al. Profound coordinated alterations of intratumoral NK cell phenotype and function in lung carcinoma. Cancer Res. (2011) 71:5412–22. doi: 10.1158/0008-5472.can-10-4179
79. Ascierto ML, Idowu MO, Zhao Y, Khalak H, Payne KK, Wang XY, et al. Molecular signatures mostly associated with NK cells are predictive of relapse free survival in breast cancer patients. J Transl Med. (2013) 11:145. doi: 10.1186/1479-5876-11-145
80. Rusakiewicz S, Semeraro M, Sarabi M, Desbois M, Locher C, Mendez R, et al. Immune infiltrates are prognostic factors in localized gastrointestinal stromal tumors. Cancer Res. (2013) 73:3499–510. doi: 10.1158/0008-5472.can-13-0371
81. Tian W, Wang L, Yuan L, Duan W, Zhao W, Wang S, et al. A prognostic risk model for patients with triple negative breast cancer based on stromal natural killer cells, tumor-associated macrophages and growth-arrest specific protein 6. Cancer Sci. (2016) 107:882–9. doi: 10.1111/cas.12964
82. Donadon M, Hudspeth K, Cimino M, Di Tommaso L, Preti M, Tentorio P, et al. Increased infiltration of natural killer and t cells in colorectal liver metastases improves patient overall survival. J Gastrointest Surg. (2017) 21:1226–36. doi: 10.1007/s11605-017-3446-6
83. Svensson MC, Warfvinge CF, Fristedt R, Hedner C, Borg D, Eberhard J, et al. The integrative clinical impact of tumor-infiltrating T lymphocytes and NK cells in relation to B lymphocyte and plasma cell density in esophageal and gastric adenocarcinoma. Oncotarget. (2017) 8:72108–26. doi: 10.18632/oncotarget.19437
84. Versluis MAC, Marchal S, Plat A, de Bock GH, van Hall T, de Bruyn M, et al. The prognostic benefit of tumour-infiltrating Natural Killer cells in endometrial cancer is dependent on concurrent overexpression of Human Leucocyte Antigen-E in the tumour microenvironment. Eur J Cancer. (2017) 86:285–95. doi: 10.1016/j.ejca.2017.09.008
85. Schleypen JS, Von Geldern M, Weiss EH, Kotzias N, Rohrmann K, Schendel DJ, et al. Renal cell carcinoma-infiltrating natural killer cells express differential repertoires of activating and inhibitory receptors and are inhibited by specific HLA class I allotypes. Int J Cancer. (2003) 106:905–12. doi: 10.1002/ijc.11321
86. Schleypen JS, Baur N, Kammerer R, Nelson PJ, Rohrmann K, Grone EF, et al. Cytotoxic markers and frequency predict functional capacity of natural killer cells infiltrating renal cell carcinoma. Clin Cancer Res. (2006) 12(3 Pt 1):718–25. doi: 10.1158/1078-0432.Ccr-05-0857
87. Mace EM, Hsu AP, Monaco-Shawver L, Makedonas G, Rosen JB, Dropulic L, et al. Mutations in GATA2 cause human NK cell deficiency with specific loss of the CD56(bright) subset. Blood. (2013) 121:2669–77. doi: 10.1182/blood-2012-09-453969
88. Poli A, Michel T, Theresine M, Andres E, Hentges F, Zimmer J. CD56bright natural killer (NK) cells: an important NK cell subset. Immunology. (2009) 126:458–65. doi: 10.1111/j.1365-2567.2008.03027.x
89. Carrega P, Morandi B, Costa R, Frumento G, Forte G, Altavilla G, et al. Natural killer cells infiltrating human nonsmall-cell lung cancer are enriched in CD56 bright CD16(-) cells and display an impaired capability to kill tumor cells. Cancer. (2008) 112:863–75. doi: 10.1002/cncr.23239
90. Kared H, Martelli S, Tan SW, Simoni Y, Chong ML, Yap SH, et al. Adaptive NKG2C+CD57+ natural killer cell and Tim-3 expression during viral infections. Front Immunol. (2018) 9:686. doi: 10.3389/fimmu.2018.00686
91. Lopez-Vergès S, Milush JM, Pandey S, York VA, Arakawa-Hoyt J, Pircher H, et al. CD57 defines a functionally distinct population of mature NK cells in the human CD56dimCD16+ NK-cell subset. Blood. (2010) 116:3865–74. doi: 10.1182/blood-2010-04-282301
92. Ménard C, Blay JY, Borg C, Michiels S, Ghiringhelli F, Robert C, et al. Natural killer cell IFN-gamma levels predict long-term survival with imatinib mesylate therapy in gastrointestinal stromal tumor-bearing patients. Cancer Res. (2009) 69:3563–9. doi: 10.1158/0008-5472.Can-08-3807
93. Xiao YS, Gao Q, Xu XN, Li YW, Ju MJ, Cai MY, et al. Combination of intratumoral invariant natural killer T cells and interferon-gamma is associated with prognosis of hepatocellular carcinoma after curative resection. PLoS ONE. (2013) 8:e70345. doi: 10.1371/journal.pone.0070345
94. Di Girolamo W, Coronato S, Portiansky E, Laguens G. Profile of immune cells in lymph nodes draining human malignant tumors. Medicina. (2008) 68:423–7.
95. Kruse PH, Matta J, Ugolini S, Vivier E. Natural cytotoxicity receptors and their ligands. Immunol Cell Biol. (2014) 92:221–9. doi: 10.1038/icb.2013.98
96. Messaoudene M, Fregni G, Enot D, Jacquelot N, Neves E, Germaud N, et al. NKp30 isoforms and NKp46 transcripts in metastatic melanoma patients: Unique NKp30 pattern in rare melanoma patients with favorable evolution. Oncoimmunology. (2016) 5:e1154251. doi: 10.1080/2162402x.2016.1154251
97. Halfteck GG, Elboim M, Gur C, Achdout H, Ghadially H, Mandelboim O. Enhanced in vivo growth of lymphoma tumors in the absence of the NK-activating receptor NKp46/NCR1. J Immunol. (2009) 182:2221–30. doi: 10.4049/jimmunol.0801878
98. Sheppard S, Schuster IS, Andoniou CE, Cocita C, Adejumo T, Kung SKP, et al. The murine natural cytotoxic receptor NKp46/NCR1 controls TRAIL protein expression in NK cells and ILC1s. Cell Rep. (2018) 22:3385–92. doi: 10.1016/j.celrep.2018.03.023
99. Zhang C, Hu Y, Shi C. Targeting natural killer cells for tumor immunotherapy. Front Immunol. (2020) 11:60. doi: 10.3389/fimmu.2020.00060
100. Boyiadzis M, Memon S, Carson J, Allen K, Szczepanski MJ, Vance BA, et al. Up-regulation of NK cell activating receptors following allogeneic hematopoietic stem cell transplantation under a lymphodepleting reduced intensity regimen is associated with elevated IL-15 levels. Biol Blood Marrow Transplant. (2008) 14:290–300. doi: 10.1016/j.bbmt.2007.12.490
Keywords: tumor-infiltrating NK cells, NK cell markers, solid tumor, prognosis, meta-analysis
Citation: Zhang S, Liu W, Hu B, Wang P, Lv X, Chen S and Shao Z (2020) Prognostic Significance of Tumor-Infiltrating Natural Killer Cells in Solid Tumors: A Systematic Review and Meta-Analysis. Front. Immunol. 11:1242. doi: 10.3389/fimmu.2020.01242
Received: 22 February 2020; Accepted: 18 May 2020;
Published: 02 July 2020.
Edited by:
Anahid Jewett, University of California, Los Angeles, United StatesReviewed by:
Daniel Olive, Aix Marseille Université, FranceMallikarjun Bidarimath, Cornell University, United States
Copyright © 2020 Zhang, Liu, Hu, Wang, Lv, Chen and Shao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zengwu Shao, szwpro@163.com
†These authors have contributed equally to this work
 Shuo Zhang
Shuo Zhang Weijian Liu1†
Weijian Liu1† Songfeng Chen
Songfeng Chen Zengwu Shao
Zengwu Shao