Abstract
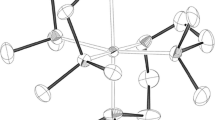
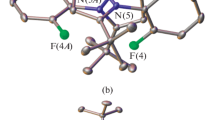
A new binuclear iron(III) oxo complex [(L)FeOFe(L)](PO2F2)4 (1) with PO2F2– counterions resulted from the oxidation of the hexafluorophosphate anion has been prepared by oxidation of an iron(II) complex with the tridentate ligand 2,6-bis(5-(4-fluorophenyl)-1H-pyrazol-3-yl)pyridine (L). According to X-ray diffraction studies, the iron(III) ions adopt the high-spin state, as in the previously described iron(III) oxo complex with tert-butyl substituted bis(pyrazol-3-yl)pyridine. At the same time, the presence of aromatic para-fluorophenyl substituents and \({\text{P}}{{{\text{O}}}_{2}}{\text{F}}_{2}^{ - }\) counterions in oxo complex 1 decreases the twisting of bis(pyrazol-3-yl)pyridine ligands relative to each other, thus ensuring a less distorted molecular geometry of this complex.

Similar content being viewed by others
REFERENCES
U. Schuchardt, D. Cardoso, R. Sercheli, et al., Appl. Catal. A 211, 1 (2001). https://doi.org/10.1016/S0926-860X(01)00472-0
S. Menage, J.-B. Galey, G. Hussler, et al., Angew. Chem., Int. Ed. Engl. 35, 2353 (1996). https://doi.org/10.1002/anie.199623531
M. Costas, M. P. Mehn, M. P. Jensen, and L. Que, Chem. Rev. 104, 939 (2004). https://doi.org/10.1021/cr020628n
M. Donald and Jr. Kurtz, J. Biol. Inorg. Chem. 2, 159 (1997). https://doi.org/10.1007/s007750050120
B. J. Wallar and J. D. Lipscomb, Chem. Rev. 96, 2625 (1996). https://doi.org/10.1021/cr9500489
M. A. Halcrow, Coord. Chem. Rev. 249, 2880 (2005). https://doi.org/10.1016/j.ccr.2005.03.010
M. Zikode, S. O. Ojwach, and M. P. Akerman, J. Mol. Catal. A 413, 24 (2016). https://doi.org/10.1016/j.molcata.2015.12.008
M. A. Halcrow, Coord. Chem. Rev. 253, 2493 (2009). https://doi.org/10.1016/j.ccr.2009.07.009
K. Umehara, S. Kuwata, and T. Ikariya, Inorg. Chim. Acta 413, 136 (2014). https://doi.org/10.1016/j.ica.2013.12.041
V. Sergienko and A. Churakov, Zh. Neorg. Khim. 61, 873 (2016). https://doi.org/10.7868/S0044457X16070175
H. Y. Qian, Russ. J. Coord. Chem. 43, 780 (2017). https://doi.org/10.1134/S1070328417110070
K. Umehara, S. Kuwata, and T. Ikariya, J. Am. Chem. Soc. 135, 6754 (2013). https://doi.org/10.1021/ja3122944
M. A. Halcrow, Crystals 6, 510 (2016). https://doi.org/3390/cryst6050058
Y. V. Nelyubina, A. V. Polezhaev, A. A. Pavlov, et al., Magnetochemistry 4, 46 (2018). https://doi.org/10.3390/magnetochemistry4040046
A. A. Pavlov, D. Y. Aleshin, I. A. Nikovskiy, et al., Eur. J. Inorg. Chem. 2019, 2819 (2019). https://doi.org/10.1002/ejic.201900432
M. A. Halcrow, Polyhedron 26, 3523 (2007). https://doi.org/10.1016/j.poly.2007.03.033
B. J. Cook, A. V. Polezhaev, C.-H. Chen, et al., Eur. J. Inorg. Chem. 2017, 3999 (2017). https://doi.org/10.1002/ejic.201700558
T. Shiga, M. Noguchi, H. Sato, et al., Dalton Trans. 42, 16185 (2013). https://doi.org/10.1039/C3DT51480C
D. Plaul, E. T. Spielberg, and W. Plass, Z. Anorg. Chem. 636, 1268 (2010). https://doi.org/10.1002/zaac.201000075
A. V. Polezhaev, C.-H. Chen, A. S. Kinne, et al., Inorg. Chem. 56, 9505 (2017). https://doi.org/10.1021/acs.inorgchem.7b00785
G. M. Sheldrick, Acta Cristallogr., Sect. A 71, 3 (2015). https://doi.org/10.1107/S2053273314026370
O. V. Dolomanov, L. J. Bourhis, R. J. Gildea, et al., J. Appl. Crystallogr. 42, 339 (2009). https://doi.org/10.1107/S0021889808042726
J. Korzekwa, A. Scheurer, F. W. Heinemann, and K. Meyer, Dalton Trans. 46, 13811 (2017). https://doi.org/10.1039/C7DT02947K
M. G. Freire, C. M. S. S. Neves, I. M. Marrucho, et al., J. Phys. Chem. A 114, 3744 (2010). https://doi.org/10.1021/jp903292n
M. A. Halcrow, Spin-Crossover Materials: Properties and Applications (Wiley, Oxford, UK, 2013).
S. Alvarez, J. Am. Chem. Soc. 125, 6795 (2003). https://doi.org/10.1021/ja0283450
S. Alvarez, Chem. Rev. 115, 13447 (2015). https://doi.org/10.1021/acs.chemrev.5b00537
ACKNOWLEDGMENTS
X-ray diffraction studies of metal complexes were carried out with the support of the Ministry of Science and Higher Education of the Russian Federation using scientific equipment at the Center for Molecular Studies of the Nesmeyanov Institute of Organoelement Compounds, Russian Academy of Sciences.
Funding
This work was financially supported by the Russian Science Foundation (grant no. 17-13-01456).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interest.
Additional information
Translated by V. Avdeeva
Rights and permissions
About this article
Cite this article
Nikovsky, I.A., Polezhaev, A.V., Melnikova, E.K. et al. New Iron(III) Oxo Complex with Substituted 2,6-Bis(Pyrazol-3-yl)Pyridine. Russ. J. Inorg. Chem. 65, 864–869 (2020). https://doi.org/10.1134/S0036023620060145
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036023620060145