Abstract
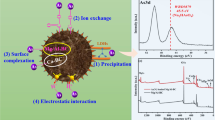
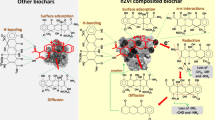
The adsorption potential of layered double hydroxides (LDH) of nickle–zinc–iron (NiZnFe) and its composites with single-wall carbon nanotubes (CNTs) and banana biochar (Bb) was investigated for divalent copper (Cu2+) removal in a batch system. Field emission scanning electron microscopy and FT-IR spectra confirmed the adsorption of Cu2+ onto LDH (NiZnFe) and its composites with Bb (LDH/Bb) and CNTs (LDH/cnt). The optimum equilibrium contact time was determined to be ~ 30 min, with LDH/Bb displaying the maximum uptake and removal efficiency (95%) for an initial Cu2+ concentration of 20 mg L−1. Pseudo-second-order kinetic models presented high R2 values (1.0) for all adsorbents, indicating good agreement between the theoretical adsorption capacities with experimental values. Multistep adsorption with both the surface and pore diffusion mechanism was suggested as well based on the intraparticle diffusion kinetic model. An optimum pH of 5.0 was considered with an increase in the uptake of Cu2+ and its removal efficiency, wherein LDH/Bb presented a greater removal efficiency and higher Cu2+ uptake compared with that of LDH (NiZnFe) and LDH/cnt. A gradual increase in Cu2+ uptake was observed in association with an increase in adsorbent dose from 0.2 to 0.5 g, with insignificant changes upon further increasing the dose of the adsorbent from 0.5 to 0.9 g. An increase in the initial Cu2+ concentrations from 10 to 100 mg L−1 resulted in a decrease in the removal efficiency, whereas Cu2+ uptake increased almost linearly in the Cu2+ concentration range of 10–60 mg L−1. Results of experimental data fitting using the Langmuir and Freundlich isotherm models suggest a dominance of monolayer adsorption, although multilayer adsorption appears to occur onto adsorbents with heterogeneous surfaces. Notably, chemisorption was also proposed to occur owing to the values of mean free energy of adsorption falling in the 8–16-kJ mol−1 range, as calculated using the Dubinin–Radushkevich isotherm model. Importantly, the use of the SIP isotherm model indicated LDH/Bb to exhibit higher energy of adsorption and degree of heterogeneity than other two adsorbents. Thus, biochar or CNTs composited NiZnFe-LDH could serve as efficient adsorbents for Cu2+ removal from wastewater streams.







Similar content being viewed by others
References
Abtahi, M., Mesdaghinia, A., Saeedi, R., & Nazmara, S. (2013). Biosorption of As(III) and As(V) from aqueous solutions by brown macroalga Colpomenia sinuosa biomass: kinetic and equilibrium studies. Desalination and Water Treatment, 51(16–18), 3224–3232. https://doi.org/10.1080/19443994.2012.749034.
Al-Ghouti, M. A., Li, J., Salamh, Y., Al-Laqtah, N., Walker, G., & Ahmad, M. N. M. (2010). Adsorption mechanisms of removing heavy metals and dyes from aqueous solution using date pits solid adsorbent. Journal of Hazardous Materials, 176(1–3), 510–520. https://doi.org/10.1016/j.jhazmat.2009.11.059.
Amin, M., Alazba, A., & Shafiq, M. (2015). Adsorptive removal of reactive black 5 from wastewater using bentonite clay: isotherms, kinetics and thermodynamics. Sustainability, 7(11), 15302–15318. https://doi.org/10.3390/su71115302.
Amin, M. T., Alazba, A. A., & Shafiq, M. (2018). Removal of copper and lead using banana biochar in batch adsorption systems: isotherms and kinetic studies. Arabian Journal for Science and Engineering, 1–12. https://doi.org/10.1007/s13369-017-2934-z.
Areco, M. M., & Afonso, M. d. S. (2010). Copper, zinc, cadmium and lead biosorption by Gymnogongrus torulosus. Thermodynamics and kinetics studies. Colloids and Surfaces B: Biointerfaces, 81(2), 620–628. https://doi.org/10.1016/j.colsurfb.2010.08.014.
Ayawei, N., Osikoya, A. O., Ekubo, A. T., Wankasi, D., & Dikio, E. D. (2015). Adsorption of copper(II) from aqueous solution by Mg/Fe-layered double hydroxide. Asian Journal of Chemistry, 27(12), 4436–4442. https://doi.org/10.14233/ajchem.2015.19166.
Barakat, M. A. (2011). New trends in removing heavy metals from industrial wastewater. Arabian Journal of Chemistry, 4(4), 361–377. https://doi.org/10.1016/j.arabjc.2010.07.019.
Barka, N., Qourzal, S., Assabbane, A., Nounah, A., & Ait-Ichou, Y. (2011). Removal of reactive yellow 84 from aqueous solutions by adsorption onto hydroxyapatite. Journal of Saudi Chemical Society, 15(3), 263–267. https://doi.org/10.1016/j.jscs.2010.10.002.
Bhatnagar, A., Minocha, A. K., & Sillanpää, M. (2010). Adsorptive removal of cobalt from aqueous solution by utilizing lemon peel as biosorbent. Biochemical Engineering Journal, 48(2), 181–186. https://doi.org/10.1016/j.bej.2009.10.005.
Cavani, F., Trifirò, F., & Vaccari, A. (1991). Hydrotalcite-type anionic clays: preparation, properties and applications. Catalysis Today, 11(2), 173–301. https://doi.org/10.1016/0920-5861(91)80068-K.
Constantino, C., Gardner, M., Comber, S. D. W., Scrimshaw, M. D., & Ellor, B. (2015). The impact of tertiary wastewater treatment on copper and zinc complexation. Environmental Technology, 36(22), 2863–2871. https://doi.org/10.1080/09593330.2015.1050072.
Eshaq, G., Rabie, A. M., Bakr, A. A., Mady, A. H., & ElMetwally, A. E. (2016). Cr(VI) adsorption from aqueous solutions onto Mg–Zn–Al LDH and its corresponding oxide. Desalination and Water Treatment, 57(43), 20377–20387. https://doi.org/10.1080/19443994.2015.1110840.
Foo, K. Y., & Hameed, B. H. (2012). Preparation, characterization and evaluation of adsorptive properties of orange peel based activated carbon via microwave induced K2CO3 activation. Bioresource Technology, 104, 679–686. https://doi.org/10.1016/j.biortech.2011.10.005.
Gimbert, F., Morin-Crini, N., Renault, F., Badot, P.-M., & Crini, G. (2008). Adsorption isotherm models for dye removal by cationized starch-based material in a single component system: error analysis. Journal of Hazardous Materials, 157(1), 34–46. https://doi.org/10.1016/j.jhazmat.2007.12.072.
Goh, K.-H., Lim, T.-T., & Dong, Z. (2008). Application of layered double hydroxides for removal of oxyanions: a review. Water Research, 42(6), 1343–1368. https://doi.org/10.1016/j.watres.2007.10.043.
Günay, A., Arslankaya, E., & Tosun, İ. (2007). Lead removal from aqueous solution by natural and pretreated clinoptilolite: adsorption equilibrium and kinetics. Journal of Hazardous Materials, 146(1–2), 362–371. https://doi.org/10.1016/j.jhazmat.2006.12.034.
Guo, Y., Zhu, Z., Qiu, Y., & Zhao, J. (2012). Adsorption of arsenate on Cu/Mg/Fe/La layered double hydroxide from aqueous solutions. Journal of Hazardous Materials, 239–240, 279–288. https://doi.org/10.1016/j.jhazmat.2012.08.075.
Huang, X.-Y., Mao, X.-Y., Bu, H.-T., Yu, X.-Y., Jiang, G.-B., & Zeng, M.-H. (2011). Chemical modification of chitosan by tetraethylenepentamine and adsorption study for anionic dye removal. Carbohydrate Research, 346(10), 1232–1240. https://doi.org/10.1016/j.carres.2011.04.012.
Huang, D., Liu, C., Zhang, C., Deng, R., Wang, R., Xue, W., et al. (2019). Cr(VI) removal from aqueous solution using biochar modified with Mg/Al-layered double hydroxide intercalated with ethylenediaminetetraacetic acid. Bioresource Technology, 276, 127–132. https://doi.org/10.1016/j.biortech.2018.12.114.
Järup, L. (2003). Hazards of heavy metal contamination. British Medical Bulletin, 68(1), 167–182. https://doi.org/10.1093/bmb/ldg032.
Johnson, P. D., Girinathannair, P., Ohlinger, K. N., Ritchie, S., Teuber, L., & Kirby, J. (2008). Enhanced removal of heavy metals in primary treatment using coagulation and flocculation. Water Environment Research: a Research Publication of the Water Environment Federation, 80(5), 472–479.
Karapinar, N., & Donat, R. (2009). Adsorption behaviour of Cu2+ and Cd2+ onto natural bentonite. Desalination, 249(1), 123–129. https://doi.org/10.1016/j.desal.2008.12.046.
Karnib, M., Kabbani, A., Holail, H., & Olama, Z. (2014). Heavy metals removal using activated carbon, silica and silica activated carbon composite. Energy Procedia, 50, 113–120. https://doi.org/10.1016/j.egypro.2014.06.014.
Khobragade, M. U., & Pal, A. (2015). Adsorptive removal of Cu(II) and Ni(II) from single-metal, binary-metal, and industrial wastewater systems by surfactant-modified alumina. Journal of Environmental Science and Health. Part A, Toxic/Hazardous Substances & Environmental Engineering, 50(4), 385–395. https://doi.org/10.1080/10934529.2015.987535.
Kul, A. R., & Koyuncu, H. (2010). Adsorption of Pb(II) ions from aqueous solution by native and activated bentonite: Kinetic, equilibrium and thermodynamic study. Journal of Hazardous Materials, 179(1), 332–339. https://doi.org/10.1016/j.jhazmat.2010.03.009.
Kumar, P. S., Ramakrishnan, K., & Gayathri, R. (2010). Removal of nickel(II) from aqueous solutions by ceralite IR 120 cationic exchange resins. Journal of Engineering Science and Technology, 5(2), 232–243.
Lei, C., Zhu, X., Zhu, B., Jiang, C., Le, Y., & Yu, J. (2017). Superb adsorption capacity of hierarchical calcined Ni/Mg/Al layered double hydroxides for Congo red and Cr(VI) ions. Journal of Hazardous Materials, 321, 801–811. https://doi.org/10.1016/j.jhazmat.2016.09.070.
Malik, P. K. (2003). Use of activated carbons prepared from sawdust and rice-husk for adsorption of acid dyes: a case study of acid yellow 36. Dyes and Pigments, 56(3), 239–249. https://doi.org/10.1016/S0143-7208(02)00159-6.
Meili, L., Lins, P. V., Zanta, C. L. P. S., Soletti, J. I., Ribeiro, L. M. O., Dornelas, C. B., et al. (2019). MgAl-LDH/biochar composites for methylene blue removal by adsorption. Applied Clay Science, 168, 11–20. https://doi.org/10.1016/j.clay.2018.10.012.
Mekonnen, M. M., & Hoekstra, A. Y. (2016). Four billion people facing severe water scarcity. Science Advances, 2(2), e1500323. https://doi.org/10.1126/sciadv.1500323.
Naddafi, K., Rastkari, N., Nabizadeh, R., Saeedi, R., Gholami, M., & Sarkhosh, M. (2016). Adsorption of 2,4,6-trichlorophenol from aqueous solutions by a surfactant-modified zeolitic tuff: batch and continuous studies. Desalination and Water Treatment, 57(13), 5789–5799. https://doi.org/10.1080/19443994.2015.1005693.
O’Connell, D. W., Birkinshaw, C., & O’Dwyer, T. F. (2008). Heavy metal adsorbents prepared from the modification of cellulose: a review. Bioresource Technology, 99(15), 6709–6724. https://doi.org/10.1016/j.biortech.2008.01.036.
Prakash, N., Sudha, P. N., & Renganathan, N. G. (2011). Copper and cadmium removal from synthetic industrial wastewater using chitosan and nylon 6. Environmental Science and Pollution Research International, 19(7), 2930–2941. https://doi.org/10.1007/s11356-012-0801-8.
Qdais, H. A., & Moussa, H. (2004). Removal of heavy metals from wastewater by membrane processes: a comparative study. Desalination, 164(2), 105–110. https://doi.org/10.1016/S0011-9164(04)00169-9.
Qiu, H., Lv, L., Pan, B., Zhang, Q., Zhang, W., & Zhang, Q. (2009). Critical review in adsorption kinetic models. Journal of Zhejiang University. Science. A, 10(5), 716–724. https://doi.org/10.1631/jzus.A0820524.
Raji, F., & Pakizeh, M. (2013). Study of Hg(II) species removal from aqueous solution using hybrid ZnCl2-MCM-41 adsorbent. Applied Surface Science, 282, 415–424. https://doi.org/10.1016/j.apsusc.2013.05.145.
Rashed, M. N. (2013). Adsorption technique for the removal of organic pollutants from water and wastewater. Organic Pollutants - Monitoring, Risk and Treatment. https://doi.org/10.5772/54048.
Rojas, R. (2014). Copper, lead and cadmium removal by Ca Al layered double hydroxides. Applied Clay Science, 87, 254–259. https://doi.org/10.1016/j.clay.2013.11.015.
Sahu, O. (2016). Treatment of industry wastewater using thermo-chemical combined processes with copper salt up to recyclable limit. International Journal of Sustainable Built Environment, 5(2), 288–300. https://doi.org/10.1016/j.ijsbe.2016.05.006.
Saiah, F. B. D., Su, B.-L., & Bettahar, N. (2009). Nickel–iron layered double hydroxide (LDH): textural properties upon hydrothermal treatments and application on dye sorption. Journal of Hazardous Materials, 165(1), 206–217. https://doi.org/10.1016/j.jhazmat.2008.09.125.
Shafiq, M., Alazba, A. A., & Amin, M. T. (2018). Removal of heavy metals from wastewater using date palm as a biosorbent: a comparative review. Sains Malaysiana, 47(1), 35–49. https://doi.org/10.17576/jsm-2018-4701-05.
Shafiq, M., Alazba, A. A., & Amin, M. T. (2019). Synthesis, characterization, and application of date palm leaf waste-derived biochar to remove cadmium and hazardous cationic dyes from synthetic wastewater. Arabian Journal of Geosciences, 12(2), 63. https://doi.org/10.1007/s12517-018-4186-y.
Shahmirzadi, M. A. A., Hosseini, S. S., & Tan, N. R. (2016). Enhancing removal and recovery of magnesium from aqueous solutions by using modified zeolite and bentonite and process optimization. Korean Journal of Chemical Engineering, 33(12), 3529–3540. https://doi.org/10.1007/s11814-016-0218-z.
Shao, M., Han, J., Wei, M., Evans, D. G., & Duan, X. (2011). The synthesis of hierarchical Zn–Ti layered double hydroxide for efficient visible-light photocatalysis. Chemical Engineering Journal, 168(2), 519–524. https://doi.org/10.1016/j.cej.2011.01.016.
Shen, Y., Zhao, X., Zhang, X., Li, S., Liu, D., & Fan, L. (2016). Removal of Cu 2+ from the aqueous solution by tartrate-intercalated layered double hydroxide. Desalination and Water Treatment, 57(5), 2064–2072. https://doi.org/10.1080/19443994.2014.981866.
Taty-Costodes, V. C., Fauduet, H., Porte, C., & Delacroix, A. (2003). Removal of Cd(II) and Pb(II) ions, from aqueous solutions, by adsorption onto sawdust of Pinus sylvestris. Journal of Hazardous Materials, 105(1), 121–142. https://doi.org/10.1016/j.jhazmat.2003.07.009.
Visa, M. (2016). Synthesis and characterization of new zeolite materials obtained from fly ash for heavy metals removal in advanced wastewater treatment. Powder Technology, 294, 338–347. https://doi.org/10.1016/j.powtec.2016.02.019.
Wang, T., Li, C., Wang, C., & Wang, H. (2018). Biochar/MnAl-LDH composites for Cu (ΙΙ) removal from aqueous solution. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 538, 443–450. https://doi.org/10.1016/j.colsurfa.2017.11.034.
Yadav, D. K., & Srivastava, S. (2017). Carbon nanotubes as adsorbent to remove heavy metal ion (Mn+7) in wastewater treatment. Materials Today: Proceedings, 4(2, part a), 4089–4094. https://doi.org/10.1016/j.matpr.2017.02.312.
Yang, J., Hou, B., Wang, J., Tian, B., Bi, J., Wang, N., et al. (2019). Nanomaterials for the removal of heavy metals from wastewater. Nanomaterials, 9(3), 424. https://doi.org/10.3390/nano9030424.
Zaghouane-Boudiaf, H., Boutahala, M., & Arab, L. (2012). Removal of methyl orange from aqueous solution by uncalcined and calcined MgNiAl layered double hydroxides (LDHs). Chemical Engineering Journal, 187, 142–149. https://doi.org/10.1016/j.cej.2012.01.112.
Zhang, M., Gao, B., Yao, Y., & Inyang, M. (2013). Phosphate removal ability of biochar/MgAl-LDH ultra-fine composites prepared by liquid-phase deposition. Chemosphere, 92(8), 1042–1047. https://doi.org/10.1016/j.chemosphere.2013.02.050.
Acknowledgments
The authors thank the Deanship of Scientific Research and RSSU at King Saud University for their technical support.
Funding
The project was financially supported by the Vice Deanship of Research Chairs, King Saud University, Riyadh, KSA.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Shafiq, M., Alazba, A.A. & Amin, M.T. Adsorption of Divalent Copper Ions from Synthetic Wastewater Using Layered Double Hydroxides (NiZnFe) and Its Composites with Banana Biochar and Carbon Nanotubes. Water Air Soil Pollut 231, 346 (2020). https://doi.org/10.1007/s11270-020-04732-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-020-04732-6