Abstract
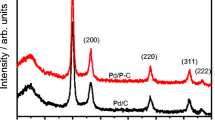
The glucose oxidation reaction, at Pd on unsupported carbon nano-onions (Pd/CNOs), has been studied by using physical and electrochemical characterization techniques. The rotating disk slurry electrode (RoDSE) technique was used for the Pd electrodeposition at the carbon support. The Pd/CNO catalyst was compared with the optimized RoDSE-prepared Pd/Vulcan XC-72R nanoflake catalyst for the glucose oxidation reaction by using different physical and electrochemical characterization techniques. Powder XRD analysis verified the effect of the carbon support material on the Pd crystallinity and size nanoparticles. Raman spectroscopy and X-ray photoelectron spectroscopy were used to understand the chemical structure of each carbon support before and after the Pd electrodeposition. The surface area and porosity of both Pd/C catalysts and their respective carbon substrates were investigated using N2 adsorption analysis. Transmission electron microscopy images established the morphology and the sizes of the Pd/CNOs, obtaining a large dispersion of nanoparticles with an average diameter size between 35 and 40 nm, with smaller particles in the range between 2 and 10 nm. Finally, cyclic voltammetry (CV) and linear sweep voltammetry (LSV) were used to compare the electrocatalytic activity of Pd/C, Vulcan XC-72R, and CNOs, for the glucose oxidation reaction in alkaline media. The results indicate that Pd/CNOs has the capacity to oxidize glucose in the normal glucose range between 5 and 8 mM, the normal range of glucose in human blood.









Similar content being viewed by others
References
National Diabetes Data G (1979) Classification and diagnosis of diabetes mellitus and other categories of glucose intolerance. Diabetes 28(12):1039–1057
Alberti KGMM, Zimmet PZ (1998) Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus. Provisional report of a WHO consultation. Diabet Med 15:539–553, 7
Toghill Richard KC (2010) Electrochemical non-enzymatic glucose sensor: a perspective and an evaluation. 5:1246–1301
Clark LC Jr, Lyons C (1962) Electrode systems for continuous monitoring in cardiovascular surgery. Ann N Y Acad Sci 102:29–45
Wang J (2008) Electrochemical glucose biosensors. Chem Rev 108(2):814–825
Park S, Boo H, Chung TD (2006) Electrochemical non-enzymatic glucose sensors. Anal Chim Acta 556(1):46–57
Updike SJ, Hicks GP (1967) The enzyme electrode. Nature 214(5092):986–988
Guilbault GG, Lubrano GJ (1973) An enzyme electrode for the amperometric determination of glucose. Anal Chim Acta 64(3):439–455
Reitz E, Jia W, Gentile M et al (2008) CuO nanospheres based nonenzymatic glucose sensor. Electroanal An Int J Devoted to Fundam Pract Asp Electroanal 20:2482–2486
Zhu Z, Garcia-Gancedo L, Flewitt AJ, Xie H, Moussy F, Milne WI (2012) A critical review of glucose biosensors based on carbon nanomaterials: carbon nanotubes and graphene. Sensors 12(5):5996–6022
Naik KK, Gangan A, Chakraborty B, Nayak SK, Rout CS (2017) Enhanced nonenzymatic glucose-sensing properties of electrodeposited NiCo2O4–Pd nanosheets: experimental and DFT investigations. ACS Appl Mater Interfaces 9(28):23894–23903
Hwang D-W, Lee S, Seo M, Chung TD (2018) Recent advances in electrochemical non-enzymatic glucose sensors–a review. Anal Chim Acta 1033:1–34
Lee W-C, Kim K-B, Gurudatt NG, Hussain KK, Choi CS, Park DS, Shim YB (2019) Comparison of enzymatic and non-enzymatic glucose sensors based on hierarchical Au-Ni alloy with conductive polymer. Biosens Bioelectron 130:48–54
Qiu F, Wang D, Zhu Q, Zhu L, Tong G, Lu Y, Yan D, Zhu X (2014) Real-time monitoring of anticancer drug release with highly fluorescent star-conjugated copolymer as a drug carrier. Biomacromolecules 15(4):1355–1364
Wang Y, Chen J, Zhou C, Zhou L, Kong Y, Long H, Zhong S (2014) A novel self-cleaning, non-enzymatic glucose sensor working under a very low applied potential based on a Pt nanoparticle-decorated TiO2 nanotube array electrode. Electrochim Acta 115:269–276
Niu X, Lan M, Zhao H, Chen C (2013) Well-dispersed Pt cubes on porous Cu foam: high-performance catalysts for the electrochemical oxidation of glucose in neutral media. Chem Eur J 19(29):9534–9541
Lee S, Lee J, Park S, Boo H, Kim HC, Chung TD (2018) Disposable non-enzymatic blood glucose sensing strip based on nanoporous platinum particles. Appl Mater Today 10:24–29
Gougis M, Tabet-Aoul A, Ma D, Mohamedi M (2014) Laser synthesis and tailor-design of nanosized gold onto carbon nanotubes for non-enzymatic electrochemical glucose sensor. Sensors Actuators B Chem 193:363–369
Huo H, Guo C, Li G, Han X, Xu C (2014) Reticular-vein-like Cu@Cu2O/reduced graphene oxide nanocomposites for a non-enzymatic glucose sensor. RSC Adv 4(39):20459–20465
Luo B, Li X, Yang J, Li X, Xue L, Li X, Gu J, Wang M, Jiang L (2014) Non-enzymatic electrochemical sensors for the detection of hydrogen peroxide based on Cu2O/Cu nanocomposites. Anal Methods 6(4):1114–1120
Huang J, Dong Z, Li Y, Li J, Tang W, Yang H, Wang J, Bao Y, Jin J, Li R (2013) MoS2 nanosheet functionalized with Cu nanoparticles and its application for glucose detection. Mater Res Bull 48(11):4544–4547
Wang X, Ge C, Chen K, Zhang YX (2018) An ultrasensitive non-enzymatic glucose sensors based on controlled petal-like CuO nanostructure. Electrochim Acta 259:225–232
Gao A, Zhang X, Peng X, Wu H, Bai L, Jin W, Wu G, Hang R, Chu PK (2016) In situ synthesis of Ni(OH)2/TiO2 composite film on NiTi alloy for non-enzymatic glucose sensing. Sensors Actuators B Chem 232:150–157
Wang Z, Hu Y, Yang W, Zhou M, Hu X (2012) Facile one-step microwave-assisted route towards Ni nanospheres/reduced graphene oxide hybrids for non-enzymatic glucose sensing. Sensors 12(4):4860–4869
Ye J-S, Chen C-W, Lee C-L (2015) Pd nanocube as non-enzymatic glucose sensor. Sensors Actuators B Chem 208:569–574
Lu L-M, Li H-B, Qu F, Zhang XB, Shen GL, Yu RQ (2011) In situ synthesis of palladium nanoparticle–graphene nanohybrids and their application in nonenzymatic glucose biosensors. Biosens Bioelectron 26(8):3500–3504
Li Y, Niu X, Tang J, Lan M, Zhao H (2014) A comparative study of nonenzymatic electrochemical glucose sensors based on Pt-Pd nanotube and nanowire arrays. Electrochim Acta 130:1–8
Chai D, Zhang X, Chan SH, Li G (2019) Facile aqueous phase synthesis of Pd3Cu–B/C catalyst for enhanced glucose electrooxidation. J Taiwan Inst Chem Eng 95:139–146
Moore AD, Holmes SM, Roberts EPL (2012) Evaluation of porous carbon substrates as catalyst supports for the cathode of direct methanol fuel cells. RSC Adv 2(4):1669–1674
Habibi B, Mohammadyari S (2015) Facile synthesis of Pd nanoparticles on nano carbon supports and their application as an electrocatalyst for oxidation of ethanol in alkaline media: the effect of support. Int J Hydrog Energy 40(34):10833–10846
Rodriguez-Reinoso F (1998) The role of carbon materials in heterogeneous catalysis. Carbon 36(3):159–175
Antolini E (2009) Carbon supports for low-temperature fuel cell catalysts. Appl Catal B Environ 88(1-2):1–24
Plonska-Brzezinska ME, Echegoyen L (2013) Carbon nano-onions for supercapacitor electrodes: recent developments and applications. J Mater Chem A 1(44):13703–13714
Palkar A, Melin F, Cardona CM et al (2007) Reactivity differences between carbon nano onions (CNOs) prepared by different methods. Chem Asian J 2(5):625–633
Santiago D, Rodríguez-Calero GG, Palkar A, Barraza-Jimenez D, Galvan DH, Casillas G, Mayoral A, Jose-Yacamán M, Echegoyen L, Cabrera CR (2012) Platinum electrodeposition on unsupported carbon nano-onions. Langmuir 28(49):17202–17210
Cunci L, Velez CA, Perez I, Suleiman A, Larios E, José-Yacamán M, Watkins JJ, Cabrera CR (2014) Platinum electrodeposition at unsupported electrochemically reduced nanographene oxide for enhanced ammonia oxidation. ACS Appl Mater Interfaces 6(3):2137–2145
Plonska-Brzezinska ME, Dubis AT, Lapinski A, Villalta-Cerdas A, Echegoyen L (2011) Electrochemical properties of oxidized carbon nano-onions: DRIFTS-FTIR and Raman spectroscopic analyses. ChemPhysChem 12(14):2659–2668
Kruk M, Jaroniec M (2001) Gas adsorption characterization of ordered organic− inorganic nanocomposite materials. Chem Mater 13(10):3169–3183
Kruk M, Jaroniec M, Bereznitski Y (1996) Adsorption study of porous structure development in carbon blacks. J. Colloid Interface Sci 182(1):282–288
Kruk M, Li Z, Jaroniec M, Betz WR (1999) Nitrogen adsorption study of surface properties of graphitized carbon blacks. Langmuir 15(4):1435–1441
Velez C, Corchado-Garcia J, Rojas-Pérez A et al (2017) Manufacture of Pd/carbon Vulcan XC-72R nanoflakes catalysts for ethanol oxidation reaction in alkaline media by RoDSE method. J Electrochem Soc 164(14):D1015–D1021
Wang F, Yang L, Tang Q, Guo Y, Hao G (2013) Synthesis of Pd/XC-72 catalysts by a facile glutamate-mediated method for solvent-free selective oxidation of DL-sec-phenethylalcohol. Catal Sci Technol 3(5):1246–1252
Hiura H, Ebbesen TW, Tanigaki K, Takahashi H (1993) Raman studies of carbon nanotubes. Chem Phys Lett 202(6):509–512
Jawhari T, Roid A, Casado J (1995) Raman spectroscopic characterization of some commercially available carbon black materials. Carbon 33(11):1561–1565
Blyth RIR, Buqa H, Netzer FP, Ramsey MG, Besenhard JO, Golob P, Winter M (2000) XPS studies of graphite electrode materials for lithium ion batteries. Appl Surf Sci 167(1-2):99–106
Khodabakhshi S, Fulvio PF, Andreoli E (2020) Carbon black reborn: structure and chemistry for renewable energy harnessing. Carbon 162:604–649
Chmiola J, Yushin G, Gogotsi Y, Portet C, Simon P, Taberna PL (2006) Anomalous increase in carbon capacitance at pore sizes less than 1 nanometer. Science 313(5794):1760–1763
Heller A (1999) Implanted electrochemical glucose sensors for the management of diabetes. Annu Rev Biomed Eng 1(1):153–175
U.S. Department of Health and Human Services Food and Drug Administration Center for Devices and Radiological Health (2016) Self-monitoring blood glucose test systems for over-the-counter use
Funding
This work was supported by the National Science Foundation NSF-PREM: Center for Interfacial Electrochemistry of Energy Materials (CiE2M) grant number DMR-1827622. The use of the Cornell Center for Materials Research Shared Facilities, which is supported through the NSF MRSEC grant number DMR-1719875, is greatly appreciated. LE wishes to thank the National Science Foundation, NSF-CHE 1801317, for generous support for this work and the Robert A Welch Foundation for the endowed chair, grant number AH-0033.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Vélez, C.A., Soto-Pérez, J.J., Corchado-García, J. et al. Glucose oxidation reaction at palladium-carbon nano-onions in alkaline media. J Solid State Electrochem 25, 207–217 (2021). https://doi.org/10.1007/s10008-020-04729-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-020-04729-5