Abstract
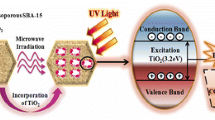
Silica SBA-16, an efficient photocatalyst support with cubic (bottle-ink) mesoporous structure, large specific surface area, large pore volume and uniform pore size and titanium oxide (TiO2), a semiconductor compound as the active site were synthesized facilely via one-pot method and photocatalysts denoted as x%TiO2/SBA-16 where x = 2, 5, 10 and 20, in order to examine their photocatalytic ability for phenol degradation in different experimental conditions such as different phenol concentration in water (ppm), various pH of the solution and amount of applied photocatalyst (g/l). In order to confirm the correct structure of the SBA-16 support and prepared photocatalysts, various types of characterization analyses such as N2 adsorption/desorption, X-ray diffraction (XRD), TEM, SEM–EDX, UV–VIS and FT-IR were applied. Based on the employed analyses, it can be claimed that the SBA-16 support and synthesized photocatalysts demonstrated mesoporous structures with cubic pores that are in agreement with reliable literature. After carrying out all of the experimental factors for the phenol degradation process, it was found that the 10%TiO2/SBA-16 photocatalyst in the specific experimental conditions including pH 7, phenol concentration of 100 ppm and 1 g/l photocatalyst dosage achieved the highest performance which was approximately 90% degradation of phenol. Hence these mentioned conditions were chosen as the optimum experimental conditions. COD and TOC tests were employed to study the final products of the process and their results showed that the 10%TiO2/SBA-16 photocatalyst was able to reduce the COD and TOC of phenol solution up to 80% and 85%, respectively, means that the major amount of phenol converted to water and CO2 that are the final products of phenol degradation process.









Similar content being viewed by others
Data availability
All of data and results are presented in the text.
References
Cheremisinoff PN (2019) Handbook of water and wastewater treatment technology. Routledge, Abingdon
Bamuza-Pemu EE (2014) Photocatalytic degradation of phenolic compounds and algal metabolites in water. University of Pretoria, Pretoria
Alharbi OM, Khattab RA, Ali I (2018) Health and environmental effects of persistent organic pollutants. J Mol Liq 263:442–453
Rahmani A, Samadi M, Enayati Moafagh A (2008) Investigation of photocatalytic degradation of phenol by UV/TiO2 process in aquatic solutions. J Res Health Sci 8(2):55–60
Rodríguez M (2003) Fenton and UV-vis based advanced oxidation processes in wastewater treatment: degradation, mineralization and biodegradability enhancement. Universitat de Barcelona, Barcelona
Wang L, Lv Q, An M, Liu Z, Song Y, Zhou Y, Li J, Xu J (2018) Identification of toxic substances in phenol-acetone wastewater on activated sludge and selective toxicity removal performance with ferrous pretreatment. Environ Sci Pollut Res 25(20):19628–19634
Busca G, Berardinelli S, Resini C, Arrighi L (2008) Technologies for the removal of phenol from fluid streams: a short review of recent developments. J Hazard Mater 160(2–3):265–288
Srivastava VC, Swamy MM, Mall ID, Prasad B, Mishra IM (2006) Adsorptive removal of phenol by bagasse fly ash and activated carbon: equilibrium, kinetics and thermodynamics. Colloids Surf A 272(1–2):89–104
Gonzalez JA, Macedo E, Soares M, Medina A (1986) Liquid-liquid equilibria for ternary systems of water-phenol and solvents: data and representation with models. Fluid Phase Equilib 26(3):289–302
Pinto R, Lintomen L, Luz L Jr, Wolf-Maciel M (2005) Strategies for recovering phenol from wastewater: thermodynamic evaluation and environmental concerns. Fluid Phase Equilib 228:447–457
Dąbrowski A, Podkościelny P, Hubicki Z, Barczak M (2005) Adsorption of phenolic compounds by activated carbon—a critical review. Chemosphere 58(8):1049–1070
Kondo M, Sato H (1994) Treatment of wastewater from phenolic resin process by pervaporation. Desalination 98(1–3):147–154
Hoshi M, Kogure M, Saitoh T, Nakagawa T (1997) Separation of aqueous phenol through polyurethane membranes by pervaporation. J Appl Polym Sci 65(3):469–479
Mavukkandy MO, Zaib Q, Arafat HA (2018) CNT/PVP blend PVDF membranes for the removal of organic pollutants from simulated treated wastewater effluent. J. Environ Chem Eng 6(5):6733–6740
Kosari M, Golmohammadi M, Towfighi J, Ahmadi SJ (2018) Decomposition of tributhyl phosphate at supercritical water oxidation conditions: Non-catalytic, catalytic, and kinetic reaction studies. J Supercrit Fluids 133:103–113
Cherni D, Ayedi S, Jaouali I, Moussa N, Nsib MF (2020) Preparation of solar/visible-light active TiO2 photocatalysts with carboxylic acids for the degradation of phenol. Reac Kinet Mech Catal 1–12
Shen Y-H (2002) Removal of phenol from water by adsorption–flocculation using organobentonite. Water Res 36(5):1107–1114
Mukherjee S, Kumar S, Misra AK, Fan M (2007) Removal of phenols from water environment by activated carbon, bagasse ash and wood charcoal. Chem Eng J 129(1–3):133–142
Hu L, Yang X, Dang S (2011) An easily recyclable Co/SBA-15 catalyst: heterogeneous activation of peroxymonosulfate for the degradation of phenol in water. Appl Catal B 102(1–2):19–26
Wu H, Fu Y, Guo C, Li Y, Jiang N, Yin C (2018) Electricity generation and removal performance of a microbial fuel cell using sulfonated poly (ether ether ketone) as proton exchange membrane to treat phenol/acetone wastewater. Biores Technol 260:130–134
Zouzelka R, Remzova M, Brabec L, Rathousky J (2018) Photocatalytic performance of porous TiO2 layers prepared by quantitative electrophoretic deposition from organic solvents. Appl Catal B 227:70–78
Daskalaki VM, Antoniadou M, Li Puma G, Kondarides DI, Lianos P (2010) Solar light-responsive Pt/CdS/TiO2 photocatalysts for hydrogen production and simultaneous degradation of inorganic or organic sacrificial agents in wastewater. Environ Sci Technol 44(19):7200–7205
Kondarides DI, Patsoura A, Verykios XE (2010) Anaerobic photocatalytic oxidation of carbohydrates in aqueous Pt/TiO2 suspensions with simultaneous production of hydrogen. J Adv Oxid Technol 13(1):116–123
Tahir M, Amin NS (2013) Advances in visible light responsive titanium oxide-based photocatalysts for CO2 conversion to hydrocarbon fuels. Energy Convers Manag 76:194–214
Tahir M, Amin NS (2013) Recycling of carbon dioxide to renewable fuels by photocatalysis: prospects and challenges. Renew Sustain Energy Rev 25:560–579
Lin H, Huang C, Li W, Ni C, Shah SI, Tseng Y-H (2006) Size dependency of nanocrystalline TiO2 on its optical property and photocatalytic reactivity exemplified by 2-chlorophenol. Appl Catal B 68(1–2):1–11
Durgakumari V, Subrahmanyam M, Rao KS, Ratnamala A, Noorjahan M, Tanaka K (2002) An easy and efficient use of TiO2 supported HZSM-5 and TiO2+ HZSM-5 zeolite combinate in the photodegradation of aqueous phenol and p-chlorophenol. Appl Catal A 234(1–2):155–165
Tryba B, Morawski A, Inagaki M (2003) Application of TiO2-mounted activated carbon to the removal of phenol from water. Appl Catal B 41(4):427–433
Hosseini S, Borghei S, Vossoughi M, Taghavinia N (2007) Immobilization of TiO2 on perlite granules for photocatalytic degradation of phenol. Appl Catal B 74(1–2):53–62
Yang L, Jiang Z, Lai S, Jiang C, Zhong H (2014) Synthesis of titanium containing SBA-15 and its application for photocatalytic degradation of phenol. Int J Chem Eng 2014
Lee H, Kannan P, Al Shoaibi A, Srinivasakannan C (2019) Phenol degradation catalyzed by metal oxide supported porous carbon matrix under UV irradiation. J Water Process Eng 31:100869
Sun H, Tang Q, Du Y, Liu X, Chen Y, Yang Y (2009) Mesostructured SBA-16 with excellent hydrothermal, thermal and mechanical stabilities: Modified synthesis and its catalytic application. J Colloid Interface Sci 333(1):317–323
Ma J, Qiang L, Tang X, Li H (2010) A simple and rapid method to directly synthesize TiO2/SBA-16 with different TiO2 loading and its photocatalytic degradation performance on rhodamine B. Catal Lett 138(1–2):88–95
Ma J, Xu S, Chu J, Xue J, Tang J, Qiang L (2017) Effect of metal-support interaction on the structural and enhanced photocatalytic performance of mesoporous M-TiO 2/SBA-16 (M= Ag and Fe). J Porous Mater 24(1):45–54
Shah AT, Li B, Abdalla ZEA (2009) Direct synthesis of Ti-containing SBA-16-type mesoporous material by the evaporation-induced self-assembly method and its catalytic performance for oxidative desulfurization. J Colloid Interface Sci 336(2):707–711
Li W, Yue Q, Deng Y, Zhao D (2013) Ordered mesoporous materials based on interfacial assembly and engineering. Adv Mater 25(37):5129–5152
Zhang S, Muratsugu S, Ishiguro N, Tada M (2013) Ceria-doped Ni/SBA-16 catalysts for dry reforming of methane. ACS Catal 3(8):1855–1864
Lukens WW, Schmidt-Winkel P, Zhao D, Feng J, Stucky GD (1999) Evaluating pore sizes in mesoporous materials: a simplified standard adsorption method and a simplified Broekhoff—de Boer method. Langmuir 15(16):5403–5409
Liu G, Wang K, Hoivik N, Jakobsen H (2012) Progress on free-standing and flow-through TiO2 nanotube membranes. Sol Energy Mater Sol Cells 98:24–38
Makuła P, Pacia M, Macyk W (2018) How to correctly determine the band gap energy of modified semiconductor photocatalysts based on UV–Vis spectra. ACS Publications, Washington
Augugliaro V, Palmisano L, Sclafani A, Minero C, Pelizzetti E (1988) Photocatalytic degradation of phenol in aqueous titanium dioxide dispersions. Toxicol Environ Chem 16(2):89–109
Pardeshi S, Patil A (2008) A simple route for photocatalytic degradation of phenol in aqueous zinc oxide suspension using solar energy. Sol Energy 82(8):700–705
Naumov S (2009) Hysteresis phenomena in mesoporous materials. Verlag nicht ermittelbar
Fan C, Do D, Nicholson D (2011) On the cavitation and pore blocking in slit-shaped ink-bottle pores. Langmuir 27(7):3511–3526
Kim Y-S, Guo X-F, Kim G-J (2009) Highly active new chiral Co (III) salen catalysts immobilized by electrostatic interaction with sulfonic acid linkages on ordered mesoporous SBA-16 silica. Chem Commun 28:4296–4298
Sarkar SM, Ali ME, Rahman ML, Yusoff MM (2014) Preparation of mesoporous SBA-16 silica-supported biscinchona alkaloid ligand for the asymmetric dihydroxylation of olefins. J Nanomater 2014:1
Ijadpanah-Saravy H, Safari M, Khodadadi-Darban A, Rezaei A (2014) Synthesis of titanium dioxide nanoparticles for photocatalytic degradation of cyanide in wastewater. Anal Lett 47(10):1772–1782
Ye Du, Bai Y, Liu Y, Guo Y, Cai X, Feng Q (2016) One-pot synthesis of [111]-/{010} facets coexisting anatase nanocrystals with enhanced dye-sensitized solar cell performance. ChemistrySelect 1(21):6632–6640
Viezbicke BD, Patel S, Davis BE, Birnie DP III (2015) Evaluation of the Tauc method for optical absorption edge determination: ZnO thin films as a model system. Phys Status Solidi B 252(8):1700–1710
Ullah S, Ferreira-Neto EP, Pasa AA, Alcântara CC, Acuna JJ, Bilmes SA, Ricci MLM, Landers R, Fermino TZ, Rodrigues-Filho UP (2015) Enhanced photocatalytic properties of core@ shell SiO2@ TiO2 nanoparticles. Appl Catal B 179:333–343
Gärtner M, Dremov V, Müller P, Kisch H (2005) Bandgap widening of titania through semiconductor support interactions. ChemPhysChem 6(4):714–718
Kibombo HS, Peng R, Rasalingam S, Koodali RT (2012) Versatility of heterogeneous photocatalysis: synthetic methodologies epitomizing the role of silica support in TiO2 based mixed oxides. Catal Sci Technol 2(9):1737–1766
Ohno T, Numakura K, Itoh H, Suzuki H, Matsuda T (2009) Control of the quantum size effect of TiO2–SiO2 hybrid particles. Mater Lett 63(20):1737–1739
Andrade GF, Soares DCF, de Sousa Almeida RK, Sousa EMB (2012) Mesoporous silica SBA-16 functionalized with alkoxysilane groups: preparation, characterization, and release profile study. J Nanomater 2012:75
Zhang X, Yang H, Huo Y, Li J, Ma J, Ma J (2016) Cu (I)-Functionalized SBA-16: an efficient catalyst for the synthesis of α-ketoamides under moderate conditions. Dalton Trans 45(21):8972–8983
Rasalingam S, Peng R, Koodali RT (2014) Removal of hazardous pollutants from wastewaters: applications of TiO2-SiO2 mixed oxide materials. J Nanomater 2014:10
Khatamian M, Oskoui MS, Darbandi M (2013) Synthesis and characterization of aluminium-free ZSM-5 type chromosilicates in different alkaline systems and investigation of their pore structures. Microporous Mesoporous Mater 182:50–61
Zhang J, Zhang H, Yang X, Huang Z, Cao W (2011) Study on the deactivation and regeneration of the ZSM-5 catalyst used in methanol to olefins. J Nat Gas Chem 20(3):266–270
Luttrell T, Halpegamage S, Tao J, Kramer A, Sutter E, Batzill M (2014) Why is anatase a better photocatalyst than rutile?-Model studies on epitaxial TiO2 films. Sci Rep 4:4043
Kitazawa S-i, Choi Y, Yamamoto S, Yamaki T (2006) Rutile and anatase mixed crystal TiO2 thin films prepared by pulsed laser deposition. Thin Solid Films 515(4):1901–1904
Akbal F, Onar AN (2003) Photocatalytic degradation of phenol. Environ Monit Assess 83(3):295–302
Chiou C-H, Wu C-Y, Juang R-S (2008) Influence of operating parameters on photocatalytic degradation of phenol in UV/TiO2 process. Chem Eng J 139(2):322–329
Ahmed S, Rasul M, Martens WN, Brown R, Hashib M (2010) Heterogeneous photocatalytic degradation of phenols in wastewater: a review on current status and developments. Desalination 261(1–2):3–18
Davydov L, Reddy EP, France P, Smirniotis PG (2001) Transition-metal-substituted titania-loaded MCM-41 as photocatalysts for the degradation of aqueous organics in visible light. J Catal 203(1):157–167
Daabool FS, Hussein FH (2016) Photocatalytic degradation of phenol using TiO2 active carbon. Asian J Chem 28(2):455
Tian L, Liu H, Gao Y (2012) Degradation and adsorption of rhodamine B and phenol on TiO2/MCM-41. Kinet Catal 53(5):554–559
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Gholizadeh, F., Dianat, M.J. & Izadbakhsh, A. Photocatalytic degradation of phenol using silica SBA-16 supported TiO2. Reac Kinet Mech Cat 130, 1171–1192 (2020). https://doi.org/10.1007/s11144-020-01817-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11144-020-01817-5