Abstract
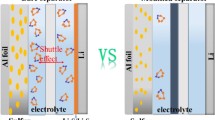
Lithium-sulfur batteries have been attracting considerable research attention due to their high energy densities and low costs. However, one of their main challenges is the undesired shuttling of polysulfides, causing rapid capacity degradation. Herein, we report the first example of sulfiphilic VSe2 ultrafine nanocrystals immobilized on nitrogen-doped graphene to modify the battery separator for alleviating the shuttling problem. VSe2 nanocrystals provide numerous active sites for chemisorption of polysulfides as well as benefit the nucleation and growth of Li2S. Furthermore, the kinetic reactions are accelerated which is confirmed by higher exchange current density and higher lithium ion diffusion coefficient. And the first-principles calculations further show that the exposed sulfiphilic planes of VSe2 boost the redox of Li2S. When used as separators within the lithium sulfur batteries, the cell indicates greatly enhanced electrochemical performances with excellent long cycling stability and exceptional rate capability up to 8 C. Moreover, it delivers a higher areal capacity of 4.04 mAh·cm−2 as well as superior cycling stability with sulfur areal loading up to 6.1 mg·cm−2. The present strategy can encourage us in engineering novel multifunctional separators for energy-storage devices.

Similar content being viewed by others
References
Bruce, P. G.; Freunberger, S. A.; Hardwick, L. J.; Tarascon, J. M. Li-O2 and Li-S batteries with high energy storage. Nat. Mater.2012, 11, 19–29.
Zhang, G.; Zhang, Z. W.; Peng, H. J.; Huang, J. Q.; Zhang, Q. A toolbox for lithium-sulfur battery research: Methods and protocols. Small Methods2017, 1, 1700134.
Hao, G. P.; Tang, C.; Zhang, E.; Zhai, P. Y.; Yin, J.; Zhu, W. C.; Zhang, Q.; Kaskel, S. Thermal exfoliation of layered metal-organic frameworks into ultrahydrophilic graphene stacks and their applications in Li-S batteries. Adv. Mater.2017, 29, 1702829.
Pan, H. L.; Chen, J. Z.; Cao, R. G.; Murugesan, V.; Rajput, N. N.; Han, K. S.; Persson, K.; Estevez, L.; Engelhard, M. H.; Zhang, J. G. et al. Non-encapsulation approach for high-performance Li-S batteries through controlled nucleation and growth. Nat. Energy2017, 2, 813–820.
Liu, D. H.; Zhang, C.; Zhou, G. M.; Lv, W.; Ling, G. W.; Zhi, L. J.; Yang, Q. H. Catalytic effects in lithium-sulfur batteries: Promoted sulfur transformation and reduced shuttle effect. Adv. Sci.2018, 5, 1700270.
Li, G. R.; Lei, W.; Luo, D.; Deng, Y. P.; Wang, D.; Chen, Z. W. 3D porous carbon sheets with multidirectional ion pathways for fast and durable lithium-sulfur batteries. Adv. Energy Mater.2018, 8, 1702381.
Zhou, Y. Y.; Hu, G. J.; Zhang, W. K., Li, Q. W.; Zhao, Z. G.; Zhao, Y.; Li, F.; Geng, F. X. Cationic two-dimensional sheets for an ultralight electrostatic polysulfide trap toward high-performance lithium-sulfur batteries. Energy Storage Mater.2017, 9, 39–46.
Liu, W.; Jiang, J. B.; Yang, K. R.; Mi, Y. Y.; Kumaravadivel, P.; Zhong, Y. R.; Fan, Q.; Weng, Z.; Wu, Z. S.; Cha, J. J. et al. Ultrathin dendrimer-graphene oxide composite film for stable cycling lithium-sulfur batteries. Proc. Natl. Acad. Sci. USA2017, 114, 3578–3583.
Mikhaylik, Y. V.; Akridge, J. R. Polysulfide shuttle study in the Li/S battery system. J. Electrochem. Soc.2004, 151, A1969–A1976.
Ji, X. L.; Lee, K. T.; Nazar, L. F. A highly ordered nanostructured carbon-sulphur cathode for lithium-sulphur batteries. Nat. Mater.2009, 8, 500–506.
Zhang, J. H.; Huang, M.; Xi, B. J.; Mi, K.; Yuan, A. H.; Xiong, S. L. Systematic study of effect on enhancing specific capacity and electrochemical behaviors of lithium-sulfur batteries. Adv. Energy Mater.2018, 8, 1701330.
Lu, H. Y.; Zhang, C.; Zhang, Y. F.; Huang, Y. P.; Liu M. K.; Liu, T. X. Simultaneous growth of carbon nanotubes on inner/outer surfaces of porous polyhedra: Advanced sulfur hosts for lithium-sulfur batteries. Nano Res.2018, 11, 6155–6166.
Mi, K.; Jiang, Y.; Feng, J. K.; Qian, Y. T.; Xiong, S. L. Hierarchical carbon nanotubes with a thick microporous wall and inner channel as efficient scaffolds for lithium-sulfur batteries. Adv. Funct. Mater.2016, 26, 1571–1579.
Qie, L.; Manthiram, A. A facile layer-by-layer approach for high-arealcapacity sulfur cathodes. Adv. Mater.2015, 27, 1694–1700.
Zhang, C. F.; Wu, H. B.; Yuan, C. Z.; Guo, Z. P.; Lou, X. W. Confining sulfur in double-shelled hollow carbon spheres for lithium-sulfur batteries. Angew. Chem., Int. Ed.2012, 51, 9592–9595.
Jayaprakash, N.; Shen, J.; Moganty, S. S.; Corona, A.; Archer, L. A. Porous hollow carbon@sulfur composites for high-power lithium-sulfur batteries. Angew. Chem., Int. Ed.2011, 50, 5904–5908.
Zheng, G. Y.; Zhang, Q. F.; Cha, J. J.; Yang, Y.; Li, W. Y.; Seh, Z. W.; Cui, Y. Amphiphilic surface modification of hollow carbon nanofibers for improved cycle life of lithium sulfur batteries. Nano Lett.2013, 13, 1265–1270.
Li, Z.; Zhang, J. T.; Lu, Y.; Lou, X. W. A pyrolyzed polyacrylonitrile/selenium disulfide composite cathode with remarkable lithium and sodium storage performances. Sci. Adv.2018, 4, eaat1687.
Fu, Y. Z.; Manthiram, A. Orthorhombic bipyramidal sulfur coated with polypyrrole nanolayers as a cathode material for lithium-sulfur batteries. J. Phys. Chem. C2012, 116, 8910–8915.
Zhou, W. D.; Yu, Y. C.; Chen, H.; DiSalvo, F. J.; Abruña, H. D. Yolk-shell structure of polyaniline-coated sulfur for lithium-sulfur batteries. J. Am. Chem. Soc.2013, 135, 16736–16743.
Li, Z.; Zhang, J. T.; Lou, X. W. Hollow carbon nanofibers filled with MnO2 nanosheets as efficient sulfur hosts for lithium-sulfur batteries. Angew. Chem., Int. Ed.2015, 54, 12886–12890.
Liang, X.; Hart, C.; Pang, Q.; Garsuch, A.; Weiss, T.; Nazar, L. F. A highly efficient polysulfide mediator for lithium-sulfur batteries. Nat. Commun.2015, 6, 5682.
Li, Z.; Guan, B. Y.; Zhang, J. T.; Lou, X. W. A compact nanoconfined sulfur cathode for high-performance lithium-sulfur batteries. Joule2017, 1, 576–587.
Lin, C.; Qu, L. B.; Li, J. T.; Cai, Z. Y.; Liu, H. Y.; He, P.; Xu, X.; Mai, L. Q. Porous nitrogen-doped carbon/MnO coaxial nanotubes as an efficient sulfur host for lithium sulfur batteries. Nano Res.2019, 12, 205–210.
Sun, Q.; Xi, B. J.; Li, J. Y.; Mao, H. Z.; Ma, X. J.; Liang, J. W.; Feng, J. K.; Xiong, S. L. Nitrogen-doped graphene-supported mixed transition-metal oxide porous particles to confine polysulfides for lithium-sulfur batteries. Adv. Energy Mater.2018, 8, 1800595.
Chen, L.; Yang, W. W.; Liu, J. G.; Zhou Y. Decorating CoSe2 hollow nanospheres on reduced graphene oxide as advanced sulfur host material for performance enhanced lithium-sulfur batteries. Nano Res.2019, 12, 2743–2748.
Yuan, Z.; Peng, H. J.; Hou, T. Z.; Huang, J. Q.; Chen, C. M.; Wang, D. W.; Cheng, X. B.; Wei, F.; Zhang, Q. Powering lithium-sulfur battery performance by propelling polysulfide redox at sulfiphilic hosts. Nano Lett.2016, 16, 519–527.
Ye, C.; Zhang, L.; Guo, C. X.; Li, D. D.; Vasileff, A.; Wang, H. H.; Qiao, S. Z. A 3D hybrid of chemically coupled nickel sulfide and hollow carbon spheres for high performance lithium-sulfur batteries. Adv. Funct. Mater.2017, 27, 1702524.
Peng, H. J.; Zhang, G.; Chen, X.; Zhang, Z. W.; Xu, W. T.; Huang, J. Q.; Zhang, Q. Enhanced electrochemical kinetics on conductive polar mediators for lithium-sulfur batteries. Angew. Chem., Int. Ed.2016, 55, 12990–12995.
Zhou, T. H.; Zhao, Y.; Zhou, G. M.; Lv, W.; Sun, P. J.; Kang, F. Y.; Li, B. H.; Yang, Q. H. An in-plane heterostructure of graphene and titanium carbide for efficient polysulfide confinement. Nano Energy2017, 39, 291–296.
Zhong, Y. R.; Yin, L. C.; He, P.; Liu, W.; Wu, Z. S.; Wang, H. L. Surface chemistry in cobalt phosphide-stabilized lithium-sulfur batteries. J. Am. Chem. Soc.2018, 140, 1455–1459.
Zhang, J. T.; Hu, H.; Li, Z.; Lou, X. W. Double-shelled nanocages with cobalt hydroxide inner shell and layered double hydroxides outer shell as high-efficiency polysulfide mediator for lithium-sulfur batteries. Angew. Chem., Int. Ed.2016, 55, 3982–3986.
Jiang, J.; Zhu, J. H.; Ai, W.; Wang, X. L.; Wang, Y. L.; Zou, C. J.; Huang, W.; Yu, T. Encapsulation of sulfur with thin-layered nickel-based hydroxides for long-cyclic lithium-sulfur cells. Nat. Commun.2015, 6, 8622.
Zhou, J. W.; Li, R.; Fan, X. X.; Chen, Y. F.; Han, R. D.; Li, W.; Zheng, J.; Wang, B.; Li, X. G. Rational design of a metal-organic framework host for sulfur storage in fast, long-cycle Li-S batteries. Energy Environ. Sci.2014, 7, 2715–2724.
Liu, J.; Qian, T.; Xu, N.; Wang, M. F.; Zhou, J. Q.; Shen, X. W.; Yan, C. L. Dendrite-free and ultra-high energy lithium sulfur battery enabled by dimethyl polysulfide intermediates. Energy Storage Mater.2020, 24, 265–271.
Chen, W.; Qian, T.; Xiong, J.; Xu, N.; Liu, J.; Zhou, J. Q.; Shen, X.; Yang, T. Z.; Chen, Y.; Yan, C. L. A new type of multifunctional polar binder: Toward practical application of high energy lithium sulfur batteries. Adv. Mater.2017, 29, 1605160.
Yuan, H.; Huang, J. Q.; Peng, H. J.; Titirici, M. M.; Xiang, R.; Chen, R. J.; Liu, Q. B.; Zhang, Q. A review of functional binders in lithium-sulfur batteries. Adv. Energy Mater.2018, 8, 1802107.
Niu, C. Q.; Liu, J.; Qian, T.; Shen, X. W.; Zhou, J. Q.; Yan, C. L. Single lithium-ion channel polymer binder for stabilizing sulfur cathodes. Natl. Sci. Rev.2020, 7, 315–323.
Zhuang, T. Z.; Huang, J. Q.; Peng, H. J.; He, L. Y.; Cheng, X. B.; Chen, C. M.; Zhang, Q. Rational integration of polypropylene/graphene oxide/nafion as ternary-layered separator to retard the shuttle of polysulfides for lithium-sulfur batteries. Small2016, 12, 381–389.
Su, Y. S.; Manthiram, A. Lithium-sulphur batteries with a microporous carbon paper as a bifunctional interlayer. Nat. Commun.2012, 3, 1166.
Chung, S. H.; Han, P.; Singhal, R.; Kalra, V.; Manthiram, A. Electrochemically stable rechargeable lithium-sulfur batteries with a microporous carbon nanofiber filter for polysulfide. Adv. Energy Mater.2015, 5, 1500738.
Yao, H. B.; Yan, K.; Li, W. Y.; Zheng, G. Y.; Kong, D. S.; Seh, Z. W.; Narasimhan, V. K.; Liang, Z.; Cui, Y. Improved lithium-sulfur batteries with a conductive coating on the separator to prevent the accumulation of inactive S-related species at the cathode-separator interface. Energy Environ. Sci.2014, 7, 3381–3390.
Liang, X.; Hart, C.; Pang, Q.; Garsuch, A.; Weiss, T.; Nazar, L. F. A highly efficient polysulfide mediator for lithium-sulfur batteries. Nat. Commun.2015, 6, 5682.
Hu, Z.; Liu, Q. N.; Chou, S. L.; Dou, S. X. Advances and challenges in metal sulfides/selenides for next-generation rechargeable sodium-ion batteries. Adv. Mater.2017, 29, 1700606.
Zhu, P.; Zhu, J. D.; Zang, J.; Chen, C.; Lu, Y.; Jiang, M. J.; Yan, C. Y.; Dirican, M.; Selvan, R. K.; Zhang, X. W. A novel bi-functional double-layer rGO-PVDF/PVDF composite nanofiber membrane separator with enhanced thermal stability and effective polysulfide inhibition for high-performance lithium-sulfur batteries. J. Mater. Chem. A2017, 5, 15096–15104.
Huang, J. Q.; Zhang, Q.; Peng, H. J.; Liu, X. Y.; Qian, W. Z.; Wei, F. Ionic shield for polysulfides towards highly-stable lithium-sulfur batteries. Energy Environ. Sci.2014, 7, 347–353.
Kong, Y. W.; An, L.; Luo, Y.; Wang, D.; Jiang, K.; Li, Q.; Fan, S.; Wang, J. Ultrathin MnO2/graphene oxide/carbon nanotube interlayer as efficient polysulfide-trapping shield for high-performance Li-S batteries. Adv. Funct. Mater.2017, 27, 1606663.
Fan, L. L.; Li, M.; Li, X. F.; Xiao, W.; Chen, Z. W.; Lu, J. Interlayer material selection for lithium-sulfur batteries. Joule2019, 3, 361–386.
Huang, J. Q.; Zhuang, T. Z.; Zhang, Q.; Peng, H. J.; Chen, C. M.; Wei, F. Permselective graphene oxide membrane for highly stable and anti-self-discharge lithium-sulfur batteries. ACS Nano2015, 9, 3002–3011.
Li, W.; Hicks-Garner, J.; Wang, J.; Liu, J.; Gross, A. F.; Sherman, E.; Graetz, J.; Vajo, J. J.; Liu, P. V2O5 polysulfide anion barrier for long-lived Li-S batteries. Chem. Mater.2014, 26, 3403–3410.
Bai, S. Y.; Liu, X. Z.; Zhu, K.; Wu, S. C.; Zhou, H. S. Metal-organic framework-based separator for lithium-sulfur batteries. Nat. Energy2016, 7, 16094.
Zhang, Z. P.; Niu, J. J.; Yang, P. F.; Gong, Y.; Ji, Q. Q.; Shi, J. P.; Fang, Q. Y.; Jiang, S. L.; Li, H.; Zhou, X. B. et al. Van der Waals epitaxial growth of 2D metallic vanadium diselenide single crystals and their extra-high electrical conductivity. Adv. Mater.2017, 29, 1702359.
Marcano, D. C.; Kosynkin, D. V.; Berlin, J. M.; Sinitskii, A.; Sun, Z. Z.; Slesarev, A.; Alemany, L. B.; Lu, W.; Tour, J. M. Improved synthesis of graphene oxide. ACS Nano2010, 4, 4806–4814.
Ghazi, Z. A.; He, X.; Khattak, A. M.; Khan, N. A.; Liang, B.; Iqbal, Z.; Wang, J. X.; Sin, H.; Li, L. S.; Tang, Z. Y. MoS2/celgard separator as efficient polysulfide barrier for long-life lithium-sulfur batteries. Adv. Mater.2017, 29, 1606817.
Kong, L.; Chen, J. X.; Peng, H. J.; Huang, J. Q.; Zhu, W. C.; Jin, Q.; Li, B. Q.; Zhang, X. T.; Zhang, Q. Current-density dependence of Li2S/Li2S2 growth in lithium-sulfur batteries. Energy Environ. Sci.2019, 12, 2976–2982.
Kresse, G.; Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B1996, 54, 11169–11186.
Blöchl, P. E. Projector augmented-wave method. Phys. Rev. B1994, 50, 17953–17979.
Dion, M.; Rydberg, H.; Schröder, E.; Langreth, D. C.; Lundqvist, B. I. Van der Waals density functional for general geometries. Phys. Rev. Lett.2004, 92, 246401.
Henkelman, G.; Uberuaga, B. P.; Jónsson, H. A climbing image nudged elastic band method for finding saddle points and minimum energy paths. J. Chem. Phys.2000, 113, 9901–9904.
Yang, C.; Feng, J. R.; Lv, F.; Zhou, J. H.; Lin, C. F.; Wang, K.; Zhang, Y. L.; Yang, Y.; Wang, W.; Li, J. B. et al. Metallic graphene-like VSe2 ultrathin nanosheets: Superior potassium-ion storage and their working mechanism. Adv. Mater.2018, 30, 1800036.
Yuan, H.; Peng, H. J.; Li, B. Q.; Xie, J.; Kong, L.; Zhao, M.; Chen, X.; Huang, J. Q.; Zhang, Q. Conductive and catalytic triple-phase interfaces enabling uniform nucleation in high-rate lithium-sulfur batteries. Adv. Energy Mater.2019, 9, 1802768.
Kong, L.; Chen, X.; Li, B. Q.; Peng, H. J.; Huang, J. Q.; Xie, J.; Zhang, Q. A bifunctional perovskite promoter for polysulfide regulation toward stable lithium-sulfur batteries. Adv. Mater.2018, 30, 1705219.
Fan, F. Y.; Carter, W. C.; Chiang, Y. M. Mechanism and kinetics of Li2S precipitation in lithium-sulfur batteries. Adv. Mater.2015, 27, 5203–5209.
Zhou, J. B.; Liu, X. J.; Zhu, L. Q.; Zhou, J.; Guan, Y.; Chen, L.; Niu, S. W.; Cai, J. Y.; Sun, D.; Zhu, Y. C. et al. Deciphering the modulation essence of p bands in Co-based compounds on Li-S chemistry. Joule2018, 2, 2681–2693.
Shi, H. F.; Lv, W.; Zhang, C.; Wang, D. W.; Ling, G. W.; He, Y. B.; Kang, F. Y.; Yang, Q. H. Functional carbons remedy the shuttling of polysulfides in lithium-sulfur batteries: Confining, trapping, blocking, and breaking up. Adv. Funct. Mater.2018, 28, 1800508.
Acknowledgements
The authors acknowledge the financial supports provided by the National Natural Science Foundation of China (Nos. 21871164, 21803036, and U1764258), the Taishan Scholar Project Foundation of Shandong Province (Nos. ts20190908 and ts201511004), and the National Science Foundation of Shandong Province (No. ZR2019MB024). The theoretical calculations in this work were performed on the HPC Cloud Platform of Shandong University. We also thank Anhui Kemi Machinery Technology Co., Ltd for providing Teflon-lined stainless steel autoclave.
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
Rights and permissions
About this article
Cite this article
Tian, W., Xi, B., Gu, Y. et al. Bonding VSe2 ultrafine nanocrystals on graphene toward advanced lithium-sulfur batteries. Nano Res. 13, 2673–2682 (2020). https://doi.org/10.1007/s12274-020-2909-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12274-020-2909-3