Abstract
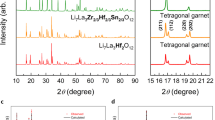
Garnet-like Al and Ga co-doped LAGLZO (Li7–3(x + y)AlxGayLa3Zr2O12) (x, y = 0.00, 0.05, 0.20, 0.25) powders were prepared in a Couette-Taylor reactor via calcination. Increased Ga content (x = 0.00, y = 0.20) was shown to be associated with improved densification and smaller grains in the sintered pellet, showing the highest ionic conductivity of 1.13 × 10−3 S cm−1 at RT. The composite solid electrolyte (CSE) sheet is composed of LAGLZO and polyethylene oxide (PEO) polymer with LiClO4 showing an ionic conductivity of 4.52 × 10−4 S cm−1 at 70 °C. However, in the LSV and symmetric cell test, the addition of Ga to the CSE sheet increased the reactivity and over-potential against lithium metal. All-solid lithium batteries (ASLBs) were fabricated using a composite cathode, CSE sheet, and Li-metal anode. The initial capacity of the ASLB slightly increased in proportion to the increasing Ga content, but the capacity retention and rate characteristics decreased as a function of charge/discharge cycles. Capacity reduction was solved by the cell design comprised of the bi-layer CSE sheets (LGLZO-based CSE/LALZO-based CSE), which suppressed the reactivity with lithium metal. Thus, by applying a LGLZO-based CSE sheet to the cathode and LALZO-based CSE sheet on lithium metal in ASLBs, the initial cell capacity with NCM 424 was improved to over 130 mAh g−1 and a capacity retention of 93% was obtained after 50 cycles.










Similar content being viewed by others
References
Goodenough JB, Park KS (2013) The Li-ion rechargeable battery: a perspective. J Am Chem Soc 135(4):1167–1176. https://doi.org/10.1021/ja3091438
Tarascon JM (2010) Key challenges in future Li-battery research. Philos Trans R Soc A 368(1923):3227–3241. https://doi.org/10.1098/rsta.2010.0112
Bernuy-Lopez C, Manalastas W, Lopez del Amo JM, Aguadero A, Aguesse F, Kilner JA (2013) Atmosphere controlled processing of Ga-substituted garnets for high Li-ion conductivity ceramics. Chem Mater 26(12):3610–3617. https://doi.org/10.1021/cm5008069
Buschmann H, Dölle J, Berendts S, Kuhn A, Bottke P, Wilkening M, Heitjans P, Senyshyn A, Ehrenberg H, Lotnyk A, Duppel V, Kienlee L, Janek J (2011) Structure and dynamics of the fast lithium ion conductor “Li7La3Zr2O12”. Phys Chem Chem Phys 13:19378–19392. https://doi.org/10.1039/C1CP22108F
Zhao P, Wen Y, Cheng J, Cao G, Jin Z, Ming H, Xu Y, Zhu X (2017) A novel method for preparation of high dense tetragonal Li7La3Zr2O12. J Power Sources 344:56–61. https://doi.org/10.1016/j.jpowsour.2017.01.088
Thangadurai V, Kaack H, Weppner W (2003) Novel fast lithium ion conduction in garnet-type Li5La3M2O12 (M = Nb, Ta). J Am Ceram Soc 86(3):437–440. https://doi.org/10.1111/j.1151-2916.2003.tb03318.x
Murugan R, Thangadurai V, Weppner W (2007) Fast lithium ion conduction in garnet-type Li7La3Zr2O12. Angew Chem Int Ed 46(41):7778–7781. https://doi.org/10.1002/anie.200701144
Awaka J, Kijima N, Hayakawa H, Akimoto J (2009) Synthesis and structure analysis of tetragonal Li7La3Zr2O12 with the garnet-related type structure. J Solid State Chem 182(8):2046–2052. https://doi.org/10.1016/j.jssc.2009.05.020
Rangasamy E, Wolfenstine J, Sakamoto J (2012) The role of Al and Li concentration on the formation of cubic garnet solid electrolyte of nominal composition Li7La3Zr2O12. Solid State Ionics 206:28–32. https://doi.org/10.1016/j.ssi.2011.10.022
Wolfenstine J, Sakamoto J, Allen JL (2012) Electron microscopy characterization of hot-pressed Al substituted Li7La3Zr2O12. J Mater Sci 47:4428–4431. https://doi.org/10.1007/s10853-012-6300-y
Düvel A, Kuhn A, Robben L, Wilkening M, Heitjans P (2012) Mechanosynthesis of solid electrolytes: preparation, characterization, and Li ion transport properties of garnet-type Al-doped Li7La3Zr2O12 crystallizing with cubic symmetry. J Phys Chem C 116(29):15192–15202. https://doi.org/10.1021/jp301193r
Wolfenstine J, Ratchford J, Rangasamy E, Sakamoto J, Allena JL (2012) Synthesis and high Li-ion conductivity of Ga-stabilized cubic Li7La3Zr2O12. Mater Chem Phys 134(2–3):571–575. https://doi.org/10.1016/j.matchemphys.2012.03.054
Wu JF, Chen EY, Yu Y, Liu L, Wu Y, Pang WK, Peterson VK, Guo X (2017) Gallium-doped Li7La3Zr2O12 garnet-type electrolytes with high lithium-ion conductivity. ACS Appl Mater Interfaces 9(2):1542–1552. https://doi.org/10.1021/acsami.6b13902
Liu Q, Geng Z, Han C, Fu Y, Li S, He YB, Kang F, Li B (2018) Challenges and perspectives of garnet solid electrolytes for all solid-state lithium batteries. J Power Sources 389:120–134. https://doi.org/10.1016/j.jpowsour.2018.04.019
Choi JH, Lee CH, Yu JH, Doh CH, Lee SM (2015) Enhancement of ionic conductivity of composite membranes for all-solid-state lithium rechargeable batteries incorporating tetragonal Li7La3Zr2O12 into a polyethylene oxide matrix. J Power Sources 274:458–463. https://doi.org/10.1016/j.jpowsour.2014.10.078
Chan CK, Yang T, Weller JM (2017) Nanostructured garnet-type Li7La3Zr2O12: synthesis, properties, and opportunities as electrolytes for Li-ion batteries. Electrochim Acta 253:268–280. https://doi.org/10.1016/j.electacta.2017.08.130
Ohta S, Seki J, Yagi Y, Kihira Y, Tani T, Asaoka T (2014) Co-sinterable lithium garnet-type oxide electrolyte with cathode for all-solid-state lithium ion battery. J Power Sources 265:40–44. https://doi.org/10.1016/j.jpowsour.2014.04.065
Choi MS, Kim HS, Kim JS, Park SJ, Lee YM, Jin BS (2014) Synthesis and electrochemical performance of high-capacity 0.34Li2MnO3·0.66LiMn0.63Ni0.24Co0.13O2 cathode materials using a Couette–Taylor reactor. Mater Res Bull 58:223–228. https://doi.org/10.1016/j.materresbull.2014.05.005
Park WK, Kim HK, Kim TY, Kim YN, Yoo SM, Kim SD, Yoon DH, Yang WS (2015) Facile synthesis of graphene oxide in a Couette–Taylor flow reactor. Carbon 83:217–223. https://doi.org/10.1016/j.carbon.2014.11.024
Langer F, Bardenhagen I, Glenneberg J, Kun R (2016) Microstructure and temperature dependent lithium ion transport of ceramic–polymer composite electrolyte for solid-state lithium ion batteries based on garnet-type Li7La3Zr2O12. Solid State Ionics 291:8–13. https://doi.org/10.1016/j.ssi.2016.04.014
Matsuda Y, Sakaida A, Sugimoto K, Mori D, Takeda Y, Yamamoto O, Imanishia N (2017) Sintering behavior and electrochemical properties of garnet-like lithium conductor Li6.25M0.25La3Zr2O12 (M: Al3+ and Ga3+). Solid State Ionics 311:69–74. https://doi.org/10.1016/j.ssi.2017.09.014
Zhang J, Zhao N, Zhang M, Li Y, Chu PK, Guo S, Di Z, Wang X, Li H (2016) Flexible and ion-conducting membrane electrolytes for solid-state lithium batteries : dispersion of garnet nanoparticles in insulating polyethylene oxide. Nano Energy 28:447–454. https://doi.org/10.1016/j.nanoen.2016.09.002
Yang SH, Kim MY, Kim DH, Jung HY, Ryu HM, Han JH, Lee MS, Kim HS (2017) Ionic conductivity of Ga-doped LLZO prepared using Couette-Taylor reactor for all-solid lithium batteries. J Ind Eng Chem 56:422–427. https://doi.org/10.1016/j.jiec.2017.07.041
Kim DH, Kim MY, Yang SH, Ryu HM, Jung HY, Ban HJ, Park SJ, Lim JS, Kim HS (2019) Fabrication and electrochemical characteristics of NCM-based all-solid lithium batteries using nano-grade garnet Al-LLZO powder. J Ind Eng Chem 71:445–451. https://doi.org/10.1016/j.jiec.2018.12.001
Qin S, Zhu X, Jiang Y, Ling M, Hu Z, Zhu J (2018) Growth of self-textured Ga3+-substituted Li7La3Zr2O12 ceramics by solid state reaction and their significant enhancement in ionic conductivity. Appl Phys Lett 112:113901. https://doi.org/10.1063/1.5019179
Rettenwander D, Geiger CA, Tribus M, Tropper P, Amthauer G (2014) A synthesis and crystal chemical study of the fast ion conductor Li7–3xGaxLa3Zr2O12 with x = 0.08 to 0.84. Inorg Chem 53(12):6264–6269. https://doi.org/10.1021/ic500803h
Jamnik J, Maier J (1999) Treatment of the impedance of mixed conductors equivalent circuit model and explicit approximate solutions. J Electrochem Soc 146:4183–4188. https://doi.org/10.1149/1.1392611
Huggins RA (2002) Simple method to determine electronic and ionic components of the conductivity in mixed conductors a review. Ionics 8:300–313. https://doi.org/10.1007/BF02376083
Rettenwander D, Redhammer G, Preishuber-Pflügl F, Cheng L, Miara L, Wagner R, Welzl A, Suard E, Doeff MM, Wilkening M, Fleig J, Amthauer G (2016) Structural and electrochemical consequences of Al and Ga cosubstitution in Li7La3Zr2O12 solid electrolytes. Chem Mater 28(7):2384–2392. https://doi.org/10.1021/acs.chemmater.6b00579
Zhang W, Nie J, Li F, Wang ZL, Sun C (2018) A durable and safe solid-state lithium battery with a hybrid electrolyte membrane. Nano Energy 45:413–419. https://doi.org/10.1016/j.nanoen.2018.01.028
Zhang Y, Chen F, Yang D, Zha W, Li JY, Shen Q, Zhang X, Zhang L (2017) High capacity all-solid-state lithium battery using cathodes with three-dimensional Li+ conductive network. J Electrochem Soc 164:A1695–A1702. https://doi.org/10.1149/2.1501707jes
Acknowledgments
This work was supported by the R&D Convergence Program (CAP-14-02-KITECH) of the National Research Council of Science & Technology of the Republic of Korea.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Jung, HY., Kim, MY., Yang, S.H. et al. Synthesis of garnet Li7–3(x + y)AlxGayLa3Zr2O12 powder by the Taylor reaction and electrochemical properties for all-solid lithium batteries. Ionics 26, 4783–4793 (2020). https://doi.org/10.1007/s11581-020-03670-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-020-03670-x