Abstract
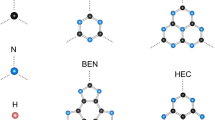
Complete optimization without geometry constraints and calculation of electronic properties of novel conic molecules such as \(\hbox {C}_{n}\hbox {H}_{n}\hbox {Ge}_{n}\hbox {H}_{n}\) and \(\hbox {C}_{n}\hbox {Ge}_{n}\hbox {H}_{n}\), with \(n = 3{-}8\), was carried out with density functional theory using B3LYP and PBE1PBE functionals with 6-31\(+\)G(d, p) and cc-pVTZ basis sets. Calculations of formation energy showed stable and peculiar geometric and electronic properties. All carbon and germanium atoms for \(\hbox {C}_{n}\hbox {H}_{n}\hbox {Ge}_{n}\hbox {H}_{n}\) compounds, which are \(\hbox {sp}^{\mathrm {3}}\)-hybridized, were located in the same plane. This finding contradicts the notions of hybridization known to date. For these new molecular compounds, quantum descriptors such as electrochemical potential (\(\mu \)), chemical hardness (\(\eta \)), electrophilicity index (\(\omega \)), dipole moment, energy gap and the shape of the molecular orbital have been calculated in addition to nucleus independent chemical shifts, polarizability and harmonic oscillator model of aromaticity which are important tools for determining the aromaticity of the studied compounds. Thus, the aim of the work is, on the one hand, to propose new stable molecular structures formed of carbon and germanium atoms, and on the other hand, to challenge our understanding of hybridization and aromaticity notion.









Similar content being viewed by others
References
Kroto H W, Heath J R, O’Brien S C, Curl R F and Smalley R E 1985 Nature 318 162
Mohan V and Datta A 2009 J. Phys. Chem. Lett. 1 136
Khajehali Z and Shamlouei H R 2018 C. R. Chim. 21 541
Dong J and Sankey O F 1999 J. Phys.: Condens. Matter 11 6129
Nijamudheen A, Bhattacharjee R, Choudhury S and Datta A 2015 J. Phys. Chem. C 119 3802
Mandal T K, Jose D, Nijamudheen A and Datta A 2014 J. Phys. Chem. C 118 12115
Graetz J, Ahn C C, Yazami R and Fultz B 2004 J. Electrochem. Soc. 151 A698M
Fuller C S and Severiens J C 1954 Phys. Rev. 96 21
Winter M and Bsenhard J O 2000 Electrochim. Acta 45 31
Ersan F, Gökçe A G and Aktürk E 2016 Appl. Surf. Sci.389 1
Ueno L T, Marim L R, Dal Pino A, Ornellas F R and Machado F B C 2006 Chem. Phys. Lett. 432 11
Krygowski T M and Szatylowicz H 2015 ChemTexts1 12
Guo L, Zheng X, Liu C, Zhou W and Zeng Z 2012 Comput. Theor. Chem. 982 17
Acikgoz S, Yungevis H, Özünal E and Şahin A 2017 J. Mater. Sci. 52 13149
Manolescu A, Macovei D, Manaila R, Grigrovici R and Pausescu P 1987 J. Non-Cryst. Solids 97&98 519
Frisch M J, Trucks G W, Schlegel H B, Scuseria G E, Robb M, Cheeseman J R et al 2004 Gaussian 03, Revision C.02 (Wallingford, CT: Gaussian, Inc.)
Frisch M J, Trucks G W, Schlegel H B, Scuseria G E, Robb M, Cheeseman J R et al 2004 Gaussian 03, Revision E.01 (Wallingford, CT: Gaussian, Inc.)
Rivière-Baudet M, Dahrouch M, Rivière P, Hussein K and Barthelat J C 2000 J. Organomet. Chem. 612 69
Boughdiri S, Hussein K, Tangour B, Dahrouch M, Rivière-Baudet M and Barthelat J C 2004 J. Organomet. Chem. 689 3279
Chattaraj P K, Sarkar U and Roy D R 2006 Chem. Rev. 106 2065
Hazarika K K, Baruah N C and Deka R C 2009 Struct. Chem. 20 1079
Parr R G, Szentpaly L and Liu S 1999 J. Am. Chem. Soc. 121 1922
Pang Q, Zhang Y, Zhang J M, Ji V and Xu K W 2011 Mater. Chem. Phys. 130 140
Zeyrek C T, Unver H, Arpacı Ö T, Polat K, İskeleli N O and Yildiz M 2015 J. Mol. Struct. 1081 22
Kassaee M Z and Aref Rad H 2010 Comput. Mater. Sci. 48 144
Böttcher C J F and Bordewijk P 1973 Theory of electric polarization 2nd edn, vol 2 (Amsterdam: Elsevier)
Kruszewski J and Krygowski T M 1972 Bull. Acad. Pol. Sci. Chim. 20 907
Kruszewski J and Krygowski T M 1972 Tetrahedron Lett. 13 3839
Krygowski T M 1993 J. Chem. Inf. Comput. Sci. 33 7
Balachandran V and Parimala K 2012 Spectrochim. Acta, Part A 96 340
Shim I, Baba M S and Ginerich K A 1998 J. Phys. Chem. A 102 10763
Brahimi M, Belmiloud Y and Kheffache D 2006 J. Mol. Struct.: THEOCHEM 759 1
Ugrinov A and Serov S C 2005 C. R. Chim. 8 1878
Siouani C, Mahtout S and Rabilloud F 2019 J. Mol. Model. 25 113
Govindarajan M, Periandy S and Carthigayen K 2012 Spectrochim. Acta, Part A 97 411
Ravikumar C, Joe I H and Jayakumar V S 2008 Chem. Phys. Lett. 460 552
Abramenko V L and Sergienko V S 2002 Russ. J. Inorg. Chem. 47 905
Zeyrek C T, Dilek N, Yıldız M and Unver H 2014 Mol. Phys. 112 2557
Schleyer P V R, Maerker C, Dransfeld A, Jiao H and Hommes N J R V E 1996 J. Am. Chem. Soc. 18 6317
Andjelkovic L, Perić M, Zlatar M, Grubišić S and Gruden-Pavlović M 2012 Tetrahedron Lett. 53 794
Acknowledgements
This work was conducted in the framework of the PRFU project, code number: B00L01UN160420190019 of MESRS of Algeria.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Elkebich, M., Zaater, S., Abtouche, S. et al. A novel carbon/germanium conic structure: theoretical study using density functional theory. Bull Mater Sci 43, 160 (2020). https://doi.org/10.1007/s12034-020-02131-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12034-020-02131-5