Anti-oxidant and anti-proliferative effect of anthocyanin enriched fractions from two Mexican wild blackberries (Rubus spp.) on HepG2 and glioma cell lines
Abstract
BACKGROUND:
Glioblastoma is the most common and deadly cancer type in the central nervous system. Following the conventional treatments in these patients, the prognosis remains poor due to high tumor recurrence. Anthocyanins from natural sources, such as blackberries (Rubus spp.), have demonstrated anti-proliferative effects on glioma cell lines. However, anthocyanins present in wild blackberries have been poorly studied on these cancer cells.
OBJECTIVE:
We aimed to determine the anthocyanins profile of two species of wild Mexican blackberries (R. liebmannii and R. palmeri), and their anti-oxidant and anti-proliferative capacities on two glioma (C6 and RG2) cell lines.
RESULTS:
We concentrated the anthocyanin content at least 26 times, revealing different proportions of these compounds in the wild blackberries. In vitro, these fractions showed anti-oxidant capacity (>10 times), while diminishing cell viability (>50%) by both Rubus fractions assayed on C6 and RG2 cells (compared to control). Also, we observed increased levels of lipid peroxidation (∼59%) by malondialdehyde formation. Results from the cell cycle and flow cytometry assays show that anthocyanins enriched fractions elicit apoptotic responses in these glioma cells.
CONCLUSIONS:
Anthocyanins present in Mexican wild blackberries constitute potential tools to develop alternative therapies to improve the survival rate in glioma patients.
1Background
Glioblastoma (GBM) is the most lethal primary brain tumor, accounting for approximately 15% of all intracranial tumors with a survival rate of 13 months after diagnosis and a 5-year survival rate of only 4–5% in clinical trials. Conventional GBM-treatments include surgery, radiotherapy, and chemotherapy; however, the prognosis for these patients remains weak due to high tumor recurrence [1–3].
In the last decade, several natural compounds have been described as alternative sources for the treatment of some human diseases, including cardiovascular disorders, inflammation, cancer, and neurodegenerative disorders [4]. Several wild edible species have been considered as economically and nutritionally important because of their anti-oxidant potential and nutritional quality. The wild edibles with significant nutraceutical value are emerging as a possible source of floral diversity conservation and development of rural communities [5, 6]. The use of wild fruit species is reported to be particularly more recurrent among food-insecure areas of rural zones. It is known that various phytochemical constituents of blackberry (Rubus spp.) fruits exhibit a wide range of biological effects on body’s health [6–8]. The most significant health benefits of berry fruits are attributed to their phenolic compounds, including flavonoids, phenolic acids, and tannins [4, 8–10]. Particularly, it has been shown that the polyphenol resveratrol elicited programmed cell death in human U251 cell line [11], whereas quercetin, a flavonoid, induced necrotic and apoptotic cell death in U138, U87, U251, and A172 cells [12]. Moreover, the co-treatment with resveratrol and quercetin arrested cell growth in the C6 line cell [13]. Similarly, extracts enriched in anthocyanins from Alba strawberry showed anti-proliferative effects on murine cancer cell lines [14]. These natural phytochemicals have been described to exhibit a wide range of biological effects, including anti-oxidant, anti-proliferative, anti-inflammatory, and anti-cancer properties [7, 8, 10].
Nonetheless, literature describing the effects on brain health’s induction of anthocyanins contained in wild species of Rubus remain scarce. Therefore, this research aimed to identify and characterize the anthocyanin content obtained from two wild species of Rubus collected in the Northwest part of Mexico, and to evaluate their potential anti-neoplastic effect on two glioma cell lines. For this purpose, total phenolic content, anthocyanins purification, anti-oxidant capacity, anti-proliferative activity, and cell-death mechanism elicited by these fractions were evaluated.
2Materials and methods
2.1Biological material and chemicals
Ripe fresh fruits of two wild blackberries were collected in the Tufted Jay Preserve, Sinaloa, Mexico (N 23°48’48.4”, O 105°50’15.1”, 2200 m a.s.l.) and freeze-dried at –80°C. These specimens were taxonomically classified following the criteria from recognized databases such as Tropicos (www.tropicos.org), World Checklist of Selected Plant Families (www.apps.kew.org), Catalogue of Life (www.catalogueoflife.org), Global Biodiversity Information Facility (www.data.gbif.org), The New York Botanical Garden Virtual Herbarium (www.data.gbif.org), JSTOR Plant Science (www.plants.jstor.org), and Biodiversity Heritage Library (www.biodiversitylibrary.org). The plant samples were identified and recorded as Rubus liebmannii Focke (IBUNAM: MEXU: 1417228) and Rubus palmeri Rybd (IBUNAM: MEXU: 1417230) at Herbarium of National Autonomous University of Mexico.
2.2Reagents
All the equipment and reagents used in this study were purchased from commercial suppliers. William’s Medium E (WME, 32551020), Dulbecco’s Modified Eagle’s Medium (DMEM, 11995073), Hepes (15630080), L-glutamine (25030169), Hanks’ Balanced Salt Solution (HBSS, 24020117), Dulbecco’s Phosphate-Buffered Saline (DPBS, 14040133) and fetal bovine serum (FBS, 11523387) were supplied by Gibcotrademark Life Technologies (Grand Island, NY, USA). Insulin (I6634), hydrocortisone (H0888), penicillin (P3032), streptomycin (P4333), bovine serum albumin (A2153), paraformaldehyde (158127), crystal violet (C0775), neutral red (N4638), (±)-6-hydroxy-2, 5, 7, 8-tetra- methylchromane-2-carboxylic acid (Trolox, 53188-07-1), 2-thiobarbituric acid (TBA, T5500), 2, 2′-azobis(2-methylpropionamidine) dihydrochloride (AAPH, 2997-94-4), fluorescein (F6377), dichlorofluorescein diacetate (DCFH-DA, 35845), cyanidin-3-glucoside chloride (PHL89616) and quercetin (Q4951) were purchased at Sigma-Aldrich, Inc. (St. Louis, MO, USA).
2.3Total phenolic content
The method to determine the total phenolic content (TPC) of fruit fractions was adjusted from Nurmi et al. [15], with some modifications. Briefly, 1.0 mg of dried samples were mixed with 0.5 mL of 1 N of Folin-Ciocalteu’s reagent and shacked for 5 min. Then, 20% Na2CO3 was added and allowed to stand for 10 min at room temperature. Immediately after this procedure, 200μL were placed in a translucid micro plate and read in a Synergy HT spectrophotometer (Biotek Instruments, VT, USA) at 765 nm. The results were expressed as mg of gallic acid equivalents per g of dry weight (mg GAE/g dw).
2.4Anthocyanins purification
The extraction, fractioning, and purification of bioactive compounds from these wild berries was done according to Cuevas-Rodríguez et al., [6]. Briefly, 100 g of freeze-dried fruits of both species were mixed with 500 mL of acidified MeOH (MeOH: ATC, 80 : 0.3%, v/w) by cold shaking at 4°C for 24 h. The crude extract (CEX) was filtered with Whatman #1 paper and concentrated by rotary evaporation (Buchi R-100, Flowill, Switzerland). The CEX was exposed to ethyl acetate (1 : 1, v/v) twice. Remained solvents were evaporated to obtain defatted CEX and non-polar fractions. The defatted CEX was exposed to the resin Amberlite® XAD-7 (Sigma-Aldrich, MO, USA) and carbohydrates were eluted with acidified water (0.3% ATC), whereas the fraction enriched in phenolic compounds was separated with acidified MeOH (MeOH: ATC, 80 : 0.3%, v/w) and concentrated with rotavapor. Finally, a column packed with the chromatographic Sephadextrademark LH-20 resin (Sigma-Aldrich, MO, USA) was used to achieve a anthocyanin enriched fraction (AEF) with acidified MeOH (MeOH: ATC, 80 : 0.3%, v/w).
2.5Characterization of anthocyanins
The anthocyanin profiling was carried out according to the procedure reported by Cuevas-Rodríguez et al. [6], with some modifications. Five mg of freeze-dried FRA was diluted in 1 mL of methanol (MeOH) and filtered through a 0.22μm nylon membrane (Milliporetrademark, Darmstadt, Germany). An LC-ESI-MS/MS 1.3 SRI system (Thermo Finnigan Corp., San Jose, CA, USA) equipped with a Supelcosil LC-18 column (150×4.6 mm, YMC-Pack ODS-AM) was used. The spray voltage was 10 kV and a capillary temperature of 250°C. Ionization was adjusted to positive mode. The flow was 200μL/min, and injection volume of 5μL was carried using a mobile phase A of 5% formic acid in a gradient corresponding to 10, 30, 60, and 10% at 0, 5, 30, and 35 min, respectively, while mobile phase B consisted in 100% MeOH. The absorbance was measured at 520 nm, and the temperature was held at 20°C. For data analysis, the MassLynxtrademark software version 4.0 (Waters Co., Milford, MA, USA) and Xcaliburtrademark Version 3.0. XCALI-97549 (Thermo Fisher Scientific Inc., Newtown, PA, USA) were used. Cyanidin commercial standards were used. Ion detection in positive mode was calibrated at m/z 100–1000 for anthocyanin fragments. Individual anthocyanin content was reported as a relative proportion percentage (%). Total anthocyanin content (TAC) of each extract (CEX, PEF, and AEF) was calculated using a cyanidin curve, and the data obtained was expressed as mg of cyanidin-3-glucoside equivalent/g of dry weight (C3GE/g dw).
2.6Oxygen radical anti-oxidant capacity (ORAC)
The anti-oxidant capacity of the obtained fruit fractions was determined, according to Ou et al. [16]. For the hydrophilic ORAC parameter, twenty-five μL of the sample was resuspended in 0.1μM PBS pH 7.4 (1 : 10, w/v) and placed in a black-walled micro plate with 150μL of N fluorescein. 25μL of a solution of 2, 2′-azobis(2-methylpropionamidine) dihydrochloride (AAPH) solubilized in PBS pH 7.0 (0.207 : 5, w/v) was added to each well, shaked at 1200 rpm for 20 s. Then, samples were read (485/538 nm of excitation/emission) at 37°C for 60 min every 5 min in a Synergy HT spectrophotometer (Biotek Instruments, VT, USA). The anti-oxidant values were quantified under a curve of Trolox, and results were expressed as μM of Trolox equivalents/100 g dry weigh (μMTE/100 g dw).
2.7HepG2, C6, and RG2 cell cultures
The human hepatocarcinoma cell line HepG2 (ATCC® HB-8065trademark) was obtained from the American Type Culture Collection. Cultured in William’s medium E (WME) with 5% FBS, 10 mM Hepes, 2 mM L-glutamine, 5μg/mL of insulin, 0.05μg/mL hydrocortisone, 50 U/mL of penicillin, 50μg/mL of streptomycin and 100μg/mL of gentamycin. On the other hand, C6 and RG2 murine glioma cell lines were also obtained from the American Type Culture Collection (ATCC® CCL-107trademark and ATCC® CRL-2433trademark, respectively). These cell lines were cultured in DMEM medium with 10% FBS, 1% penicillin-streptomycin, 1% non-essential amino acids, and 1% L-glutamine and growth at 37°C and 5% of CO2 until a confluence ≥95% in a 75 cm2 culture flask was reached [17–19].
2.8Cell viability assays
The effect of the obtained anthocyanin fractions on the viability of these cell lines was determined according to the crystal violet protocol as an indirect measurement of cell death in attached cells, as dying cells lost their adherence to the plate through cell death processes [17]. Briefly, HepG2, C6, and RG2 cells were seeded at a density of 5×104 cell/well in translucid sterile micro plates with their respective growth medium for 24 h. After the medium was removed and cells were washed three times with sterile PBS and exposed to both anthocyanins enriched fractions (AEF) and polyphenols enriched fractions (PEF) at different concentrations (0.1–1000μg/mL) for 24 h, treatments were washed as described and fixed with 50μL of 2% of paraformaldehyde/PBS for 10 min. Then, wells were washed with PBS and stained with 100μL of crystal violet (stock 1 g/L in PBS) for 30 min at 37°C. Immediately thereafter, the dye was removed and washed three times with PBS and eluted with 100μL of 10% acetic acid/PBS per well. The absorbance (570 nm) was determined in a Synergy HT spectrophotometer (Biotek Instruments, VT, USA). Results were expressed as relative cell viability percent (%) compared to control group.
2.9Cytotoxicity assay on C6 and RG2 cells
Neutral red (NR) uptake was used to assess cell viability. Cell line was exposed to the isolated fractions based on the ability of viable cells to incorporate and bind the supravital dye neutral red in the lysosomes, while death cells do not incorporate it [18]. Briefly, after 24 h of exposure to purified fractions, cells were washed with PBS, and 100μl/well of 0.33% neutral red solution (Sigma Aldrich, USA) was added to cell cultures. After an incubation period of 3 h at 25°C, cells were washed with PBS, and fixed with 1% acetic acid plus 50% absolute ethanol in PBS. This solution also elutes the incorporated NR, and the absorbance was measured at 540 nm in a micro plate reader (Cytation 3 Imagen Reader, Biotek Instruments, VT, USA). Blanks consisted of wells without cells.
2.10Cellular anti-oxidant activity on HepG2 cells
HepG2 cells were used to determine the cellular anti-oxidant activity (CAA), according to Wolfe et al. [19]. For the CAA assay, 6×104 cells/well were seeded in a black-well micro plate with 100μL of WME medium for 24 h at 37°C and 5% of CO2. The wells were then washed three times, with 100μL of sterile PBS and exposed for 1 h to 100μL of quercetin curve (0.1–250μU/mL) or samples diluted in treatment medium (WME added with 2 mM L-glutamine and 10 mM Hepes), plus 25μL of 25μM dichlorofluorescein diacetate (DCFH-DA), and 100μL of 600μM of AAPH diluted in HBSS. For the non-washing protocol, the PBS washing step was omitted. Next, the plate was read at 485/538 nm of excitation/emission at 37°C every 5 min for one hour. Each sample was performed by triplicate. Wells treated only with DCFH-DA and AAPH were considered as positive controls, whereas those treated with DCFH-DA without AAPH were considered as negative controls. CAA results were expressed as μM equivalents of quercetin per g of sample in dry weight (μM EQ/g dw).
2.11Oxidative damage to lipids in C6 and RG2 cells
Lipid peroxidation is the oxidative damage that affects cellular membranes, lipoproteins, and other molecules containing lipids under conditions of oxidative stress [20]. To determine if the purified fractions of berry fruit could exert some damage on cellular membranes and lipoproteins on C6 and RG2 cells, we determined the levels of lipid peroxidation following the procedure described by Colín-Gonz
2.12Cell-death mechanisms
2.12.1Cell cycle in C6 and RG2 cells
Propidium iodide staining of DNA is the classic approach to analyze the cell cycle in cultures since cells in the S phase exhibit more DNA than cells in the G1 phase. They will incorporate proportionally more dye and will fluoresce more brightly they have doubled their DNA content. Cells in G2 will be approximately twice as bright as cells in G1 [21]. Here, the Propidium Iodide Flow Cytometry Kit (Abcam, USA) was used following the manufacturer’s instructions to determine the effect of isolated fractions on the cell cycle of C6 and RG2 cells exposed to these fractions. In brief, C6 and RG2 cells were collected and centrifuged at 1000 g for 5 min and fixed with 70% ethanol at –20°C. Then, samples were incubated with RNase A (30 mg/mL DNase free) for 10 min at room temperature, and then samples were incubated with 80μL of PI (150 mg/mL) for 15 min at 4°C, always protected from light. Cell cycle analysis was performed by flow cytometry (BD FACS Canto II, USA) acquiring 20000 events. Collected data were analyzed by Mod Fit software [21].
2.13Annexin V/PI assay in C6 and RG2 cells
Apoptosis and necrosis are the two main forms of cell death. Annexin V binds specifically to phosphatidylserine, which is translocated from the inner part to the external membrane only in damaged cells. The differentiation between apoptotic and necrotic cells can be performed by simultaneous staining with Propidium Iodide (PI) since the cell membrane integrity excludes PI in viable and apoptotic cells.
In contrast, necrotic cells are permeable to Propidium Iodide. Thus, the Annexin V/PI assay (Molecular Probes, USA) was carried out to determine the cell cycle and the cell-death mechanisms elicited by anthocyanins on C6 and RG2 lines using commercially available kits. The method is based on the double-stain system with Annexin V conjugated to FITC and PI. Briefly, exposed cells were harvested and washed twice with cold PBS. A total of 1.0×105 cells were re-suspended in 100μL binding buffer and mixed with 5μL FITC-labeled Annexin V and 5μL PI at room temperature for 15 min in the dark. After the addition of 400μL binding buffer, apoptosis was analyzed by flow cytometry. Ten thousand events were collected for each sample for data recollection in a FACS Canto II flow cytometer (Becton Dickinson Biosciences, USA) equipped with a 20 mW laser output tuned at 488 nm, using the BD FACS Diva TM v6.1.3 software (Becton–Dickinson Biosciences). The analyzer threshold was adjusted on the forward light scatter channel cell size (FSC) to exclude noise and subcellular debris. Data were plotted as a function of fluorescence intensity of Annexin V-FITC versus PI, and the Annexin V-/PI-population was accepted as a control of healthy cells [22].
2.14Statistical analysis
Results are expressed as means±S.E.M. or S.D. Data were statistically analyzed by a two- and three-way analysis of variance (ANOVA) followed by post hoc Bonferroni’s test. A two-way ANOVA was employed to analyze the effect of purified fractions of anthocyanins on the proposed parameters seen in cell cultures. Values of p≤0.05 were considered statistical significance. Pearson’s correlation was expressed as r value. Analytical procedures were performed using the scientific statistic software GraphPad Prism 5 (GraphPad Scientific, San Diego, CA, USA).
3Results and discussion
3.1Total phenolic and anthocyanins content
The values obtained for total phenolic and anthocyanin content in the extracts from wild blackberries are given in Table 1. The total phenolic content of CEX was 15.23 mgGAE/g dw and 17.33 mgGAE/g dw for R. liebmannii and R. palmeri respectively. After the assessment of the chromatographic assay using Amberlite XAD-7, the polyphenols were concentrated to 201.03 mgGAE/g dw and 184.44 mgGAE/g dw for each specie. Finally, after passing the extracts through Sephadex LH-20 resins, the total polyphenol contents were 474.72 mgGAE/g dw and 517.10 mgGAE/g dw for each Rubus fruit, respectively.
Table 1
Total phenolic content and antioxidant capacity of extracts from R.liebmannii and R.palmeri
| Fractions | Blackberry Species | |
| R. liebmannii | R. palmeri | |
| TPC (mg GAE/g dw) | ||
| CEX | 15.23±0.6a | 17.33±0.69a |
| PEF | 201.03±6.91a | 184.44±13.32b |
| AEF | 474.72±13.4b | 517.19±28.21a |
| TAC (mg C3GE/g dw) | ||
| CEX | 6.31±0.10a | 5.58±0.16b |
| PEF | 229.83±6.47a | 218.13±3.31b |
| AEF | 758.63±69.32a | 699.54±31.74b |
| AOx | ||
| ORAC (μM ET/g dw) | ||
| CEX | 277.4±9.5a | 249.2±8.9b |
| PEF | 2921.2±97.4a | 2280.2±126.2b |
| AEF | 3755.0±108.2a | 3630.3±72.2b |
| Cellular anti-oxidant activity (CAA) | ||
| CEX | ||
| Cytotoxicity (EC50, mg/mL) | 305.85±10.02a | 297.45±3.82a |
| CAA (μM QE/g dw) | 1.24±0.10b | 1.01±0.14a |
| PEF | ||
| Cytotoxicity (EC50, mg/mL) | 86.32±3.11b | 94.22±4.09a |
| CAA (μM QE/g dw) | 14.78±1.00b | 17.44±1.14a |
| AEF | ||
| Cytotoxicity (EC50, mg/mL) | 16.89±1.32b | 19.56±0.27a |
| CAA (μM QE/g dw) | 30.63±1.25b | 36.50±1.74a |
TPC: Total phenolic content; TAC: Total anthocyanin content; AOx: Anti-oxidant capacity; mg GAE/g dw: mg of gallic acid equivalents/g of dry weight; mg C3G/g dw: mg of cyanidin-3-glucoside/g of dry weight; μM ET/g dw: μM equivalents of Trolox/g of dry weight; μM EQ/g dw:μM equivalents of quercetin/g of dry weight; EC50: half-maximal effective concentration expressed as mg of sample/mL; PEF: polyphenols enriched fraction; CEX: Crude extract; AEF: anthocyanins enriched fraction; CAA: cellular anti-oxidant activity in HepG2 cells. Mean±standard deviation, values within the same line without the same letters are significantly different (p≤0.05).
The content of anthocyanins (TAC) for each extract (CEX, PEF, and AEF) ranged from 6.31 mgC3GE/g to 5.58 mgC3GE/g, 229 mgC3GE/g to 218 mgC3GE/g, and 758 mgC3GE/g to 699 mgC3GE/g, where R. liebmannii presented the major values (p < 0.05). Strong positive correlations were found in TPC vs TAC, showing values of r = 0.9857 and r = 0.9990 for R. liebmannii and R. palmeri, respectively (Supplementary Figure 1).The results suggest that the chromatographic steps used in the investigation constitute a viable procedure to purify anthocyanins, which are the major phenolic compounds present in these fruits [6, 7].
HPLC and LC-MS analyses of anthocyanins revealed the presence of tree major anthocyanins for R. liebmannii (cyanidin-3-O-glucoside, cyanidin-3-O-rutinoside, cyanidin-3-O-(6-O-malonyl)-glucoside and four for R. palmeri (cyanidin-3-O-glucoside, cyanidin-3-O-xilosil-rutinoside, cyanidin-3-O-rutinoside, cyanidin-3-O-(6-O-malonyl)-glucoside) (Fig. 1). Furthermore, data show that the most abundant anthocyanin for R. liebmannii was cyanidin-3-O-glucoside (83.5%) and for R. palmeri cyanidin-3-O-rutinoside (45.02%). The parameters to characterize the anthocyanins content and the profile were their m/z value, UV spectrum absorbance characteristics, retention time (RT), and we compared these values with standards and following criteria previously reported in the literature [5, 6, 23]. The individual anthocyanins were verified by the LC-MS spectra, which is based on the ion charge ratio (m/z) and Rt in both genotypes, showing that the cyanidin was present in the aglycone form (m/z 287). Additionally, we found its glycosides: cyanidin-3-O-glucoside (m/z 449.02), cyanidin-3-O-(6-O-malonyl)-glucoside (m/z 535.06), cyanidin-3-O-rutinoside (m/z 595.11), and cyanidin-3-O-arabinoside (m/z 419.02). Other fragment (m/z 413.50 fragment) was observed and produced an ion of m/z = 301.15 by MS/MS, which was tentatively identified as a peonidin derivative. It is noteworthy that, in the literature the main anthocyanin reported in wild and domesticated blackberry species is cyanidin-3-glucoside [5, 6]. However, in our study the major anthocyanin detected in R. palmeri was cyanidin-3-rutinoside, whereas in R. liebmannii cyanidin-3-O-xylosil-rutinoside was not detected.
Anthocyanins are secondary metabolites synthesized by plants in response to biotic or abiotic stress. According to Wang and Stoner [10] the anthocyanins compounds are the most abundant flavonoid, constituents of fruits and vegetables such as berries, grapes, apples, purple cabbage, and corn. The diversity, type and contents of anthocyanins in plants are responsible not only for the variety of colors in fruits (from yellow to red), but also for the nutritional quality, and possible therapeutic properties as their bioactivity has been related with their chemical structure [10, 24].
Fig.1
Anthocyanins profile in R. liebmannii and R. palmeri. A) Chromatograms of Rubus anthocyanins. B) Negative mode spectrogram of main anthocyanins in R. liebmannii and R. palmeri. C) Profile and negative ions of individual anthocyanins presented in fractions of R. l iebmannii and R. palmeri; Cya-3-glu: cyanidin-3-O-glucoside; Cya-3-xyl: cyanidin-3-O-xylosil-rutinoside; Cya-3-rut: cyanidin-3-O-rutinoside; Cya-3-rut: cyanidin-3-O-rutinoside; Cya-3-mal-glu: Cyanidin-3-O-(6-malonyl)-glucoside.
3.2Anti-oxidant capacity
Values of the anti-oxidant capacity evaluated in R. liebmannii and R. palmeri by ORAC assays are presented in Table 1. This capacity in the Crude Extract (CEX) was 277.4μM TE/g for R. liebmannii and 249.2μM TE/g for R. palmeri. The fraction enriched in polyphenols content after the Amberlite XAD-7 FPA step showed values of 2921.2 and 2280.2μM TE/g of extract for R. liebmannii and R. palmeri, respectively. After purification step through Sephadex resins, the content of anthocyanins was 3755.0 and 3630.3μM TE/g of extract for R. liebmannii and R. palmeri, respectively. The TPC vs ORAC values showed strong positive correlations for R. liebmannii and R. palmeri of r = 0.8470 and r = 0.9099, respectively, while, the TAC vs ORAC only showed strong correlation for R. palmeri fractions (r = 0.8914) (Supplementary Figure 1).
These results agree with previously reported data [6] and could support the free-radical scavenging potential of anti-oxidants against peroxyl radicals. A previous report propose a strong correlation between total polyphenols, anti-oxidant activity, and anti-tumor protection of polyphenol’s intake [19]. Notably, the daily intake of anthocyanins in the U.S. diet has been estimated to be around 180 and 215 mg, whereas the consumption of other dietary flavonoids such as genistein, quercetin, and apigenin is only 20–25 mg/day [10]. Recent reports indicate that the intake of diets enriched in anthocyanins has beneficial effects against inflammation and increased gut permeability while improves colon health [25].
3.3Cellular anti-oxidant activity (CAA) on HepG2 cells
The CAA of Rubus fractions on HepG2 cells is presented in Table 1. HepG2 cell line is recommended by several research groups to evaluate CAA and insulin-dependent pathways as these cells absorb very well flavonoids such as quercetin, kaempferol, and luteolin, thus revealing that the origin of tumor tissue influences the metabolic phenotype [19, 24].
The CEX from R. liebmannii and R. palmeri has shown Medium Effective Concentration (EC50) values on HepG2 cells of 305.85 and 297.45 mg/mL, respectively (Table 1). Subsequently, the PEF indicate an EC50 of 86.32 mg/mL for R. liebmannii and 94.22 mg/mL for R. palmeri, which represents an increase of >3.5 times after a chromatographic procedure compared to the CEX. When the HepG2 cell line was exposed to AEF from R. liebmannii and R. palmeri species, we observed a strong anti-proliferative effect showing EC50 values of 16.89 and 19.56 mg/mL, respectively. AEF from both wild blackberries increased >4.1 times the cytotoxic effect in HepG2 hepatocarcinoma cell line, compared to PEF. Statistical differences (p < 0.05) were observed in all EC50 among treatments, except between CEX of each sample. The cytotoxicity observed in HepG2 cells exposed to Rubus fractions suggests a strong negative correlation (r–<0.8) between TPC and cell viability. The CAA of CEX from R. liebmannii and R. palmeri extracts were 1.24 and 1.01μM EQ/g dw, respectively; they were statistically (p < 0.05) different. Moreover, the CAA for PEF extract were 14.78 and 17.44μM EQ/g dw for R. liebmannii and R. palmeri, respectively. Meanwhile, the CAA for AEF extracts, the values found in the study were 30.63μM EQ/g dw and 36.50μM EQ/g dw for R. liebmannii and R. palmeri, respectively (Table 1). The reported CAA effects of Rubus extracts and fractions on HepG2 cells are also correlated with the purifying degree step of each extract. The CAA was increased according to polyphenols concentration value. A strong positive correlation supported was found between CAA vs TPC from R. liebmannii (r = 0.9958) and R. palmeri (r = 0.9787). The ORAC results also correlated with CAA since R. liebmannii presented an r = 0.8905 value whereas R. palmeri showed r = 0.9751 value. This could be due to the increased concentration of phenolic compounds after each purification step (Supplementary Figure 1).
Compared to the reported by Forbes-Hern
On the other hand, the CAA, compared with in vitro anti-oxidant capacity assays, provides a better prediction of bio-accessibility, bio-availability, distribution, and metabolism of bioactive dietary ingredients on cell line cultures, such as the HepG2 line [29]. Considering previous reports [4, 19], we selected the PEF and AEF extracts of each wild blackberry species to determine the anti-proliferative effect on glioma C6 and RG2 cell lines.
3.4Cytotoxicity and oxidative stress of FPA and FPS
After ingestion, anthocyanins undergo chemical changes by endogenous gastrointestinal and micro biota conditions before going through to bloodstream, but recent studies suggest that anthocyanins might survive to body setting, crossing the blood-brain barrier and exerting neuroprotective effects [30, 31]. The products of these structure bio-modifications might produce phenolic acids, anthocyanidins and/or chalcones [32]. The latter has a reversible structure to anthocyanins, depending on the pH of matrix [33]. According to the literature, the pH of glioblastoma microenvironment is acidic, therefore anthocyanins might recover their native structure and interact directly with tumor cells [34]. The new pH-sensitive drug-carriers seem to be an innovative therapy against glioma, using doses at concentrations not-allowed to cross the BBB and releasing their pharmacological substances only when involved in the cancerous matrix [34–36]. This represents a new frontier to explore novel natural compounds with neuropharmacological purposes.
Oxidative stress is strongly associated with the type development of neurodegenerative diseases and the proliferation of cancer cells, as occurs in glioma, the cancer showing the highest mortality in the central nervous system. Anthocyanins from different berry fruits exert anti-cancer activities, including anti-proliferative effects on in vitro glioma cell cultures [19]. The AEF purified from both Rubus fruits showed an anti-proliferative effect on C6 and RG2 with statistically different (p > 0.05) EC50 values ranging from 15.06 to 26.02 mg/mL (Table 2). The fraction enriched in anthocyanins isolated from R. liebmannii exhibited an EC50 = 23.25, and 30.70 mg/mL for C6 and RG2, respectively (Table 2), though the same fraction isolated from R. palmeri displayed the highest values in both cell lines, with EC50 = 34.31 and 42.22 mg/mL, respectively (Table 2). During the performance of cell viability assays by crystal violet stain, we noted that some precipitates were attached to the bottom of wells treated whit the anthocyanin fractions after the washing steps, and previous to the elution step. Therefore, we took advantage of the neutral red properties to validate the obtained data by the crystal violet technique. NR is only incorporated and metabolized in lysosomes from living cells, while dead cells do not incorporate this dye for its posterior elution. It is noteworthy; there was compatibility between the profiles of cell viability using both techniques. These values suggest that an anti-proliferative activity is present in these fractions isolated from both Rubus species on C6 and RG2 glioma cell lines even though these lines showed different susceptibility to these compounds. Complex blends of phenolic extracts from various plant materials have revealed to be excellent candidates to modulate cell proliferation on glioma cell lines. They disrupt the peroxyl chain reactions at the cell membrane surface, regulating anti-oxidant gene expression, and altering the cell cycle [37, 38]. Moreover, it has been shown that fractions enriched in anthocyanins, as AEF, affect the plasminogen activation by inhibiting the cell migration capacity of glioma cells in in vitro assays [1], while the expression of urokinase enzyme is affected by plasminogen. Thus, the invasiveness and progression of malignant cells could be interrupted by these molecules [39].
Table 2
Cytotoxicity and lipoperoxidation in C6 and RG2 glioma cells of fractions from R liebmannii and R. palmeri
| Fraction | C6 | RG2 | ||
| R. liebmannii | R. palmeri | R. liebmannii | R. palmeri | |
| Cytotoxicity (EC50) | ||||
| PEF | 19.58±0.57b | 18.50±0.32c | 26.02±1.75a | 15.06±0.85d |
| AEF | 23.25±2.07c | 34.31±2.15b | 30.70±4.09b | 42.22±3.33a |
| Lipoperoxidation (%) | ||||
| PEF | 10.85±1.23ab | 9.46±0.98b | 11.27±0.99a | 8.63±1.31b |
| AEF | 84.81±7.15a | 59.19±4.48b | 63.34±5.55b | 48.97±3.49c |
C6 and RG2: Glioma cell lines; EC50: half-maximal effective concentration; PEF: polyphenols enriched fraction; AEF: anthocyanins enriched fraction; Mean±standard deviation, values within the same line without same letters are significantly different (p > 0.05).
Oxidative stress, in extreme conditions, elicits irreversible damage to cell structures leading to cellular death. Anthocyanins could might evoke pro-oxidant conditions depending on several factors, including chemical profile and concentration. Cytotoxic effects have been demonstrated in in vitro cell cultures via lipid peroxidation of cell membranes. As previously stated, lipid peroxidation is considered an early biomarker of oxidative damage [2]. Malondialdehyde (MDA) is a low-molecular-weight aldehyde produced, among others, by oxidation of cellular polyunsaturated fatty acids. Following the formation of MDA-thiobarbituric acid (MDA-TBA), it is possible to estimate lipid peroxidation in cells. The MDA-TBA formation in C6 and RG2 is presented in Table 2. The highest levels of MDA-TBA corresponded to the R. liebmannii FPA in RG2 cells (11.27%), being similar (p > 0.05) to the treatment in C6 line (10.85%), though the FPA of R. palmeri were significantly lower (p > 0.05) compared with values of 9.46 and 8.63% in C6 and RG2 cells, respectively. The MDA formation induced by treatments with FPS showed the highest values for C6 cells (84.81%) compared with RG2 (63.34%), both obtained with FPS from R. palmeri. Diaconeasa et al. [8] reported that anthocyanins from blueberry and blackcurrants are highly recommended for daily consumption to prevent numerous degenerative diseases. At certain concentrations, anthocyanins from red wine have shown regulatory activities on the levels of MDA in the postprandial state with beneficial effects [40]. Cirak et al. [41] demonstrated a high positive correlation among tissue samples of patients with different grades of glial tumors and levels of MDA in blood serum, concluding that MDA is a molecule capable of crossing the blood-brain barrier, being an accurate compound to detect the grade of the tumor to support other therapeuticapproaches.
3.5Cell-death mechanisms
3.5.1Cell cycle and annexin V/PI flow cytometry assays
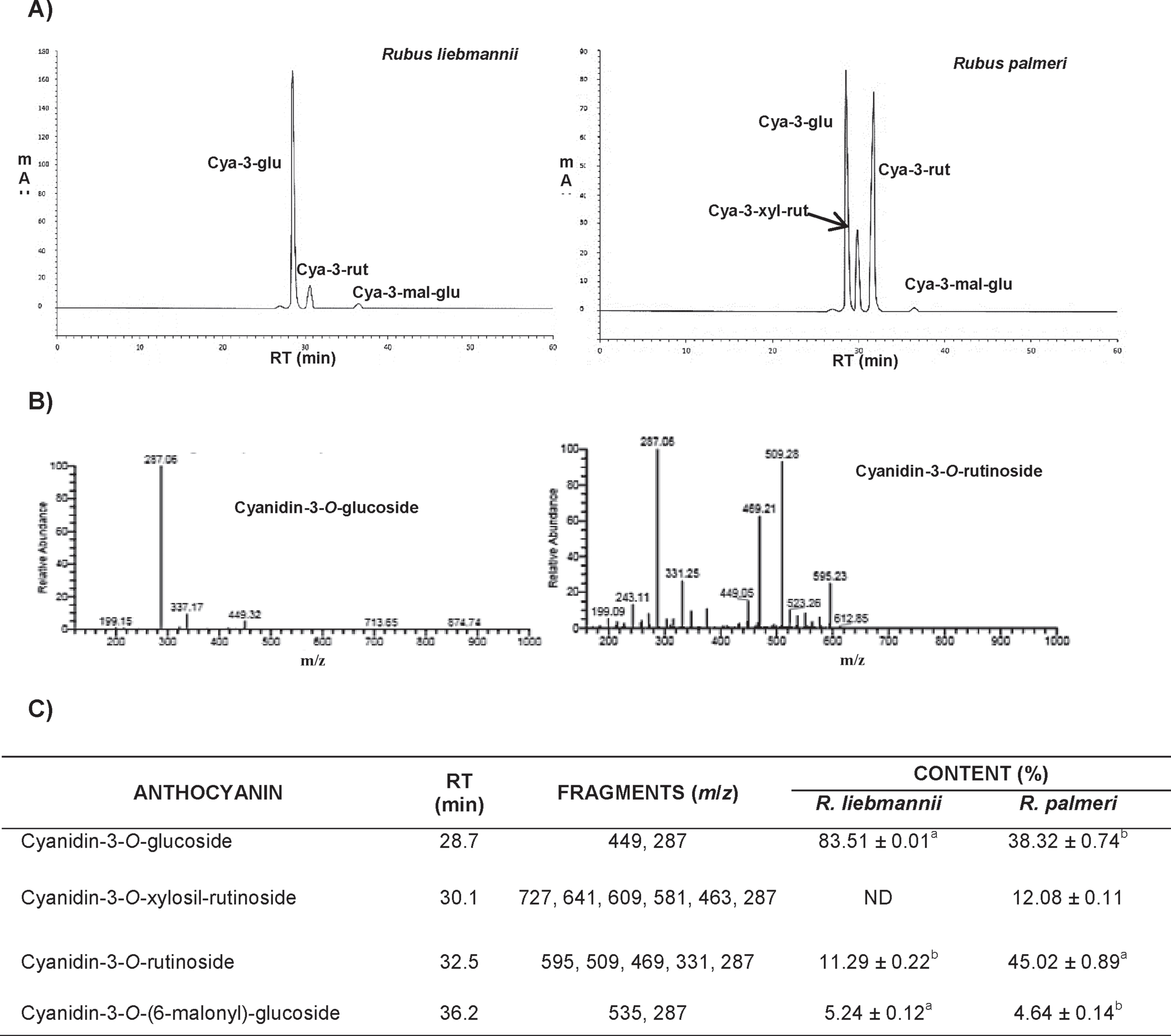
Flow cytometric cell cycle analysis revealed that the AEF (25μg/μL) from R. liebmannii arrested 76.5% of C6 cells in the G0/G1 phase, being statistically higher (p < 0.05) compared to its control group (44.7%), whereas S-phase in this cell line showed a statistical (p < 0.05) decrease of 14.0% compared to the 48.9% of the control group. Similarly, this effect was also observed when C6 cells were exposed to the AEF (35μg/μL) isolated from R. palmeri extracts, displaying a statistical increase (p < 0.05) in the G0/G1 phase from 32.4% for control group to 75.5% for AEF-treated cells. The S-phase in the control group showed a value of 57.6%, whereas in C6 cells exposed to AEF fraction this percent decreased (p < 0.05) to 18.7% (Fig. 2). When these fractions were assayed in RG2 cells, the cell cycle profile observed for the extracts obtained from both Rubus species showed slight variations compared to the control group, being the G2/M-phase the one in which the anthocyanin extract from R. palmeri showed a moderate increase, from 7.8% for the control group, to 12%.
Fig.2
Effects of R. liebmannii and R. palmeri on the cell cycle of C6 cultures (A and B) and RG2 (C and D) respectively without (Ctrl) or with the exposure to anthocyanins enriched fractions (AEF) from Rubus. The percent of cells in each phase of the cell cycle is presented for anthocyanins-treated cultures of C6 (A′ and B′) and RG2 (C′ and D′) cultures. Asterisk (*) indicates statistical difference (p < 0.05) between AEF with its respective control.
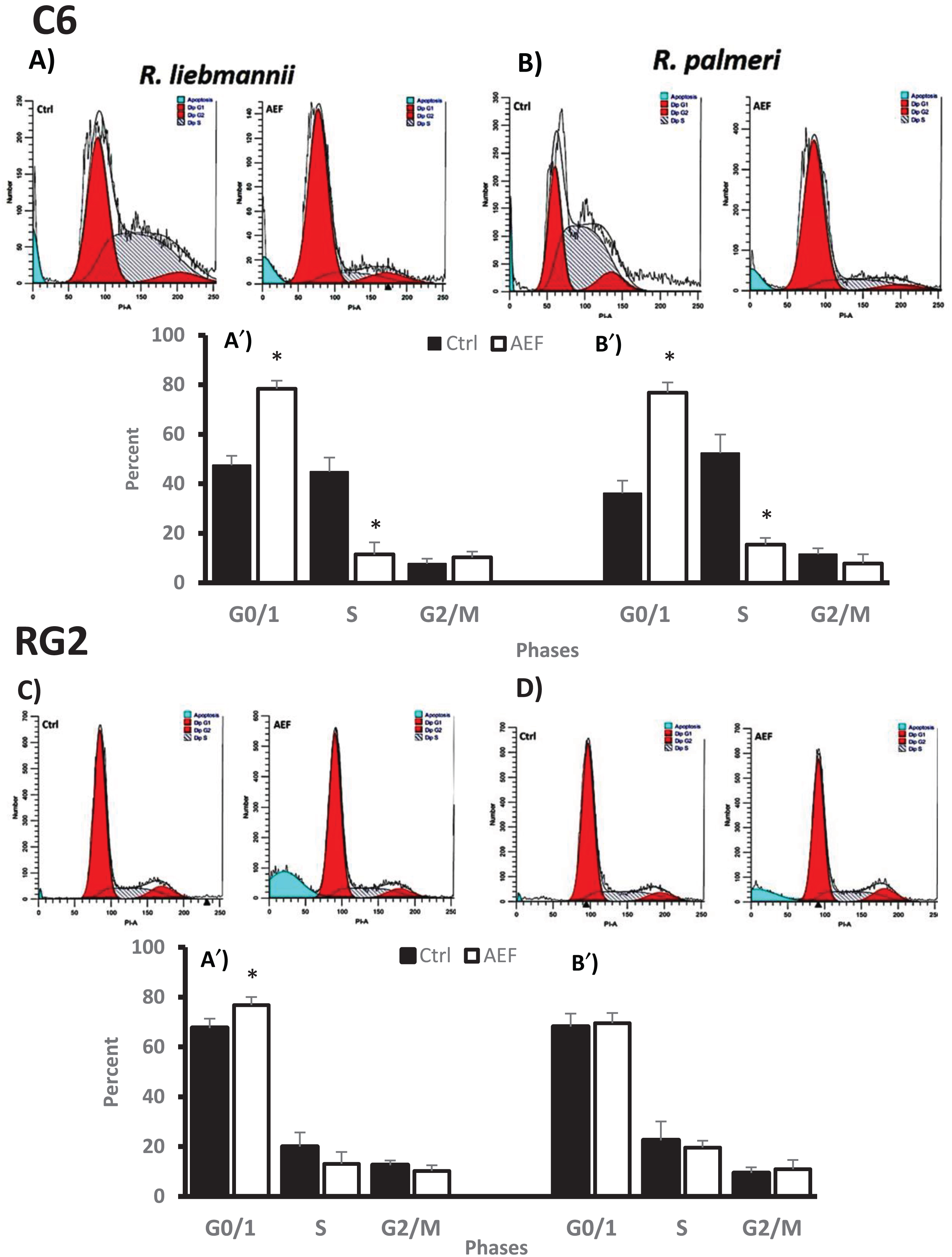
In order to elucidate the possible death mechanisms elicited by these extracts on C6 and RG2 neoplastic cell, we performed the Annexin V/PI assay by flow cytometry. As shown in Fig. 3, the control group for R. liebmannii was primarily Annexin V-FITC and PI-negative, as expected for the majority of cell populations in the healthy state (73% for C6 and 80% for RG2, p < 0.05 compared to their respective controls). After these cell lines were exposed to AEF from R. liebmannii, we observed 11% of C6 and 5% of RG2 were Annexin V-FITC positive/PI negative, indicating that they undergo early apoptosis (p < 0.05 compared to control cells). This figure also shows that 16.3% for C6 and 10.5% for RG2 were entering the end stage of apoptosis or were already death (Annexin V-FITC positive/PI positive). Finally, 27% of the population for C6 and 32% for RG2 were Annexin-FITC negative/PI positive, indicating that populations were not viable anymore.
Fig.3
Annexin V-PI double staining assay. After the treatment of C6 and RG2 cultures with R. liebmannii and R. palmeri anthocyanin extracts, cells were stained with annexin V-FITC and Propidium iodide (PI) and analyzed by flow cytometry. A) Representative image for the control group for both C6 and RG2 cultures without the treatment with anthocyanins fractions. B) Representative image for C6 cells exposed to of R. liebmannii extract (25μg/μL = EC50) and B′) R. palmeri extract (35μg/μL = EC50) respectively. C) Representative image for RG2 cells exposed to of R. liebmannii extract (30μg/μL = EC50) and C′) R. palmeri extract (40μg/μL = EC50) respectively. The percentage of apoptotic cells after the treatments with both Rubus extracts on C6 cultures D) and RG2 D′) is presented. Asterisk (*) indicates statistical difference (p < 0.05) between AEF with its respective control.
When the R. palmeri extract was assessed on these cells, we observed a decrease in the healthy population, with 48% of the whole population in early-late apoptosis and necrosis for C6 cultures, while 38.5% of the population had entered apoptotic/necrotic pathways in RG2 cultures. These results clearly demonstrate that AEF isolated from Rubus species exerted growth inhibition and elicited apoptotic effects in both tested cell lines (Fig. 3B). Other authors have also demonstrated that anthocyanins isolated from several natural sources up regulate tumor suppressor genes, alter the cell cycle, induce apoptosis in cancer cells, and reduce age-associated oxidative stress [9, 41]. Hogan and colleagues [37] reported that fractions enriched in anthocyanins isolated from Acacia sp. also present these anti-proliferative effects in C6, though literature regarding the effects of these phytochemicals in the RG2 cell line is scare. Similarly to the data presented, herein other reports also indicate that anthocyanins induced apoptosis in cancer cells via activation of redox-sensitive caspase 3-pathways [42]. Considering that anthocyanin extracts could elicit the expression of ROS-related enzymes such as super oxide dismutase, it is likely that a mechanism related to the Toll-like receptor 4 (TLR4)/nuclear factor-κB (NF-κB) and NOD-like receptor pyrin domain-containing 3 protein (NLRP3) pathways could be activated [43]. According to Sehitoglu and collaborators [9], the treatment of cancer cells with anthocyanins might up-regulate the expression of tumor suppressor genes and modulate intracellular-signaling cascades as common molecular targetsfor anthocyanins.
There are several reports indicating that some bioactive components from plants exerts protective effects on non-malignant cells. These properties could be associated whit the potential of some molecules present in these extracts to reduce intracellular ROS levels produced by normal metabolism of oxygen in organisms. In this sense, these phytochemicals could protect some biomolecules such as DNA, lipids and proteins against the damage induced by ROS. It is well known that these molecules are crucial to maintain the integrity of intracellular pathways related to anti-oxidant responses, the activation of Nrf2, the activity of anti-oxidant enzymes and other elements to preserve the energy metabolism in mitochondria, the essential cell functioning, and the continuity of the cell cycle [43].
On the other hand, it has been reported the complexity of compounds present in botanical extracts and the anti-proliferative effects of some of these phytochemicals on neoplastic cell lines mainly by eliciting apoptotic processes [8, 10, 21, 42]. It is clear that cancer cells must exhibit several and complex mechanisms to overcome microenvironmental insults and suppress the activation of apoptotic signals. The inhibition of NF-κB is crucial to induce apoptosis and has been reported that anthocyanins from Vitis coignetiae Pulliat inhibited NF-κB in colon carcinoma cells while activating p38-MAPK, which is necessary to allow the transcription of apoptotic genes [44]. Additionally, Devi et al. [45] have reported that anthocyanins increase the amount of oligonucleosomal-sized fragments, whereas anthocyanin-enriched fractions decreased the cell proliferation in B16-F10 melanoma cells and repressed pAkt expression, suggesting the involvement of HER2 signaling pathway and its phosphorylation as possible targets of anthocyanin metabolites [46, 47].
4Conclusions and perspectives
It is reported that the regular consumption of plant food sources contributes to healthy levels of radicals into the body as an effective cancer-preventing strategy. Wild species have a long history of use in traditional medicine worldwide. In Mexico, it is necessary to promote the nutraceutical and therapeutic potential that these species possess. According to previous reports, here we show that not only the phenolic content, but also the anthocyanin concentration, anti-oxidant properties, and anti-proliferative activities are different among specimens from different species. Moreover, even in individuals within the same species, differences depend on the culture conditions of the plant, including light intensity, humidity, temperature, altitude, the use of fertilizers and pesticides, wounding, infections, or other stress factors. The data reported here contribute to the improvement of nutritional and breeding programs, which have turned their focus into determining the fruit quality and strive to improve nutritional or health value to create new hybrids and cultures with enhanced levels of bioactive components.
The anthocyanins obtained from these underutilized Mexican fruits might be suitable candidates for the establishment of innovative food ingredients with potential for clinical trial approaches in patients with glioblastoma. Moreover, due to differences in the anthocyanin profile, we found that anthocyanins from R. liebmannii exhibited the highest in vitro antioxidant capacity and cellular anti-oxidant activity on HepG2 cells, as well as lipid peroxidation and induction of apoptosis on C6 and RG2 tumor cells, compared with R. palmeri anthocyanins. However, further studies are required to understand the involved molecular targets and pathways better in the induction of these effects on cancer lines. Overall, the findings depicted in this study are an essential contribution to anthocyanin research in biological studies.
Funds/Grants
This research was supported by the Universidad Autónoma de Sinaloa through PROFAPI-2014/058 program.
Conflicts of interest
The authors declare no conflicts of interest.
Author contributions
O.A.S.-V. wrote the original draft, O.A.S.-V. and M.C.-R. worked on formal analysis; M.C.-R. and J.M.-A. worked developing the used methodology; B.R.-B. was involved in the study supervision; A.S.-d.A. contributed for the manuscript conceptualization; J.M.-C. was the data curator; E.O.C.-R. and E.R.-L. conceived the original idea, working on investigation and designing the present study.
Acknowledgments
We appreciate the assistance of the Herbario Nacional from the Instituto de Biología, Universidad Nacional Autónoma de México, for the support and facilities provided to perform taxonomic classification of the collected specimens. Also, the authors express gratitude to Rocío Morales-Bárcenas from the Instituto Nacional de Cancerología for her excellent technical assistance.
Supplementary material
[1] The supplementary material is available in the electronic version of this article: https://dx.doi.org/10.3233/JBR-200566.
References
[1] | Lamy S , Lafleur S , Bédard V , Moghrabi A , Barrete S , Gingras D , Béliveau R . Anthocyanidins inhibit migration of glioblastoma cells: Structure-activity relationship and involvement of the plasmolytic system. J Cell Biochem. (2007) ;100: (1):100–11. |
[2] | Ramírez-Expósito MJ , Martínez-Martos JM . The Delicate Equilibrium between Oxidants and Antioxidants in Brain Glioma. Curr Neuropharmacol. (2019) ;17: (4):342–51. |
[3] | Thakkar JP , Dolecek TA , Horbinski C , Ostrom QT , Lightner DD , Barnholtz-Sloan JS , Villano JL . Epidemiologic and molecular prognostic review of glioblastoma. Cancer Epidemiol Biomarkers Prev. (2014) ;23: (10):1985–1996. |
[4] | Tavares L , Figueira I , McDougall GJ , Vieira HLA , Stewart D , Alves PM , Ferreira RB , Santos CN . Neuroprotective effects of digested polyphenols from wild blackberry species. Eur J Nutr. (2013) ;52: (1):225–36. |
[5] | Acosta-Montoya Ó , Vaillant F , Cozzano S , Mertz C , Pérez AM , Castro MV . Phenolic content and antioxidant capacity of tropical highland blackberry (Rubus adenotrichus Schltdl.) during three edible maturity stages. Food Chem. (2010) ;119: (4):1497–501. |
[6] | Cuevas-Rodríguez EO , Yousef GG , García-Saucedo PA , López-Medina J , Paredes-López O , Lila MA . Characterization of Anthocyanins and Proanthocyanidins in Wild and Domesticated Mexican Blackberries (Rubus spp). J Agric Food Chem. (2010) ;58: (12):7458–64. |
[7] | Sánchez-Velázquez O-A , Montes-Ávila J , Milán-Carrillo J , Reyes-Moreno C , Mora-Rochín S , Cuevas-Rodríguez E-O . Characterization of tannins from two wild blackberries (Rubus spp) by LC–ESI–MS/MS, NMR and antioxidant capacity. J Food Meas Charact. (2019) ;13: (3):2265–74. |
[8] | Diaconeasa Z , Leopold L , Rugină D , Ayvaz H , Socaciu C . Antiproliferative and antioxidant properties of anthocyanin-rich extracts from blueberry and blackcurrant juice. Int J Mol Sci. (2015) ;16: (2):2352–65. |
[9] | Sehitoglu MH , Farooqi AA , Qureshi MZ , Butt G , Aras A . Anthocyanins: Targeting of Signaling Networks in Cancer Cells. Asian Pacific J Cancer Prev. (2014) ;15: (5):2379–81. |
[10] | Wang L-S , Stoner G-D . Anthocyanins and their role in cancer prevention. Cancer Lett. (2008) ;269: (2):281–90. |
[11] | Jiang H , Zhang L , Kuo J , Kuo K , Gautam SC , Groc L , Rodriguez AI , Koubi D , Hunter TJ , Corcoran GB , Seidman MD , Levine RA . Resveratrol-induced apoptotic death in human U251 glioma cells. Mol Cancer Ther. (2005) ;4: (4):554–61. |
[12] | Siegelin MD , Reuss DE , Habel A , Rami A , von Deimling A . Quercetin promotes degradation of survivin and thereby enhances death-receptor-mediated apoptosis in glioma cells. Neuro Oncol. (2009) ;11: (2):122–31. |
[13] | Zamin LL , Filippi-Chiela EC , Dillenburg-Pilla P , Horn F , Salbego C , Lenz G . Resveratrol and quercetin cooperate to induce senescence-like growth arrest in C6 rat glioma cells. Cancer Sci. (2009) ;100: (9):1655–62. |
[14] | Mazzoni L , Giampieri F , Alvarez Suarez JM , Gasparrini M , Mezzetti B , Forbes Hernandez TY , Battino MA . Isolation of strawberry anthocyanin-rich fractions and their mechanisms of action against murine breast cancer cell lines. Food Funct. (2019) ;10: :7103–20. |
[15] | Nurmi K , Ossipov V , Haukioja E , Pihlaja K . Variation of total phenolic content and individual low-molecular-weight phenolics in foliage of mountain birch trees (Betula pubescens ssp. tortuosa). J Chem Ecol. (1996) ;22: (1):2023–40. |
[16] | Ou B , Hampsch-Woodill M , Prior RL . Development and Validation of an Improved Oxygen Radical Absorbance Capacity Assay Using Fluorescein as the Fluorescent Probe. J Agric Food Chem. (2001) ;49: (10):4619–26. |
[17] | Feoktistova M , Geserick P , Leverkus M . Crystal Violet Assay for Determining Viability of Cultured Cells. Cold Spring Harb Protoc. (2016) ;2016: (4):pdb.prot087379. |
[18] | Repetto G , del Peso A , Zurita JL . Neutral red uptake assay for the estimation of cell viability/cytotoxicity. Nat Protoc. (2008) ;3: (7):1125–31. |
[19] | Wolfe KL , Kang X , He X , Dong M , Zhang Q , Liu RH . Cellular Antioxidant Activity of Common Fruits. J Agric Food Chem. (2008) ;56: (18):8418–26. |
[20] | Colín-González AL , Becerríl H , Flores-Reyes BR , Torres I , Pinzón E , Santamaría-Del Angel D , Túnez I , Serratos I , Pedraza-Chaverrí J , Santamaría A , Maldonado PD . Acute restraint stress reduces hippocampal oxidative damage and behavior in rats: Effect of S-allyl cysteine. Life Sci. (2015) ;135: :165–72. |
[21] | Reyes-Zárate E , Sánchez-Pérez Y , Gutiérrez-Ruiz MC , Chirino YI , Osornio-Vargas ÁR , Morales-Bárcenas R , Souza-Arroyo V , García-Cuellas CM . Atmospheric particulate matter (PM10) exposure-induced cell cycle arrest and apoptosis evasion through STAT3 activation via PKCζ and Src kinases in lung cells. Environ Pollut. (2016) ;214: :646–56. |
[22] | Kotlar I , Rangel-López E , Colonnello A , Aguilera-Portillo G , Serratos IN , Galcán-Arzate S , Pedraza-Chaverri J , Túnez I , Wajner M , Santamaría A . Anandamide Reduces the Toxic Synergism Exerted by Quinolinic Acid and Glutaric Acid in Rat Brain Neuronal Cells. Neuroscience. (2019) ;401: :84–95. |
[23] | Ramirez JE , Zambrano R , Sepúlveda B , Kennelly EJ , Simirgiotis MJ . Anthocyanins and antioxidant capacities of six Chilean berries by HPLC–HR-ESI-ToF-MS. Food Chem. (2015) ;176: :106–14. |
[24] | Bobinaitė R , Viškelis P , Venskutonis PR . Variation of total phenolics, anthocyanins, ellagic acid and radical scavenging capacity in various raspberry (Rubus spp.) cultivars. Food Chem. (2012) ;132: (3):1495–501. |
[25] | Li S , Wu B , Fu W , Reddivari L . The Anti-inflammatory Effects of Dietary Anthocyanins against Ulcerative Colitis. Int J Mol Sci. (2019) ;20: (10):2588. |
[26] | Forbes-Hernández T , Gasparrini M , Afrin S , Cianciosi D , González-Paramás A , Santos-Buelga C , Mezzetti B , Quiles JL , Battino M , Giampieri F , Bompadre S . Strawberry (cv. Romina) Methanolic Extract and Anthocyanin-Enriched Fraction Improve Lipid Profile and Antioxidant Status in HepG2 Cells. Int J Mol Sci. (2017) ;18: (6):1149. |
[27] | Ali HM , Almagribi W , Al-Rashidi MN . Antiradical and reductant activities of anthocyanidins and anthocyanins, structure-activity relationship and synthesis. Food Chem. (2016) ;194: :1275–82. |
[28] | Wolfe KL , Liu RH . Structure-Activity Relationships of Flavonoids in the Cellular Antioxidant Activity Assay. J Agric Food Chem. (2008) ;56: :8404–11. |
[29] | Sefried S , Häring HU , Weigert C , Eckstein SS . Suitability of hepatocyte cell lines HepG2, AML12 and THLE-2 for investigation of insulin signalling and hepatokine gene expression. Open Biol. (2018) ;8: (10):180147. |
[30] | Helms HC , Abbott NJ , Burek M , Cecchelli R , Couraud P-O , Deli MA , Förster C , Galla HJ , Romero IA , Shusta EV , Stebbins MJ , Vandenhaute E , Weksler B , Brodin B . In vitro models of the blood–brain barrier: An overview of commonly used brain endothelial cell culture models and guidelines for their use. J Cereb Blood Flow Metab. (2016) ;36: :862–90. |
[31] | Manolescu BN , Oprea E , Mititelu M , Ruta LL , Farcasanu IC . Dietary Anthocyanins and Stroke: A Review of Pharmacokinetic and Pharmacodynamic Studies. Nutrients. (2019) ;11: (7):1479. |
[32] | David L , Danciu V , Moldovan B , Filip A . Effects of In Vitro Gastrointestinal Digestion on the Antioxidant Capacity and Anthocyanin Content of Cornelian Cherry Fruit Extract. Antioxidants. (2019) ;8: (5):114. |
[33] | Mejía-Escobar MA , Jaramillo F . Thermally and UV Stable Natural Dyes with Potential Use in Efficient Photoelectrochemical Devices. J. Renew Mat. (2015) ;3: (4):302–11. |
[34] | Zhao Y , Ren W , Zhong T , Zhang S , Huang D , Guo Y , Yao X , Wang C , Zhang WQ , Zhang X , Zhang Q . Tumor-specific pH-responsive peptide-modified pH-sensitive liposomes containing doxorubicin for enhancing glioma targeting and anti-tumor activity. J Controlled Release. (2016) ;222: ;56–66. |
[35] | Seyfoori A , Sarfarazijami S , Seyyed Ebrahimi SA . pH-responsive carbon nanotube-based hybrid nanogels as the smart anticancer drug carrier. Artif Cells Nanomed Biotechnol. (2019) ;47: (1):1437–43. |
[36] | Lakkadwala S , dos Santos Rodrigues B , Sun C , Singh J . Biodistribution of TAT or QLPVM coupled to receptor targeted liposomes for delivery of anticancer therapeutics to the brain in vitro and in vivo. Nanomed: Nanotechnol Bio-Med. (2020) ;23: :102112. |
[37] | Hogan S , Chung H , Zhang L , Li J , Lee Y , Dai Y , Zhou K . Antiproliferative and antioxidant properties of anthocyanin-rich extract from açai. Food Chem. (2010) ;118: (2):208–14. |
[38] | Pagano I , Piccinelli AL , Celano R , Campone L , Gazzerro P , De Falco E , Rastrelli L . Chemical profile and cellular antioxidant activity of artichoke by-products. Food Funct. (2016) ;7: (12):4841–50. |
[39] | Zhang X , Fei Z , Bu X , Zhen H , Zhang Z , Gu J , Chen Y . Expression and significance of urokinase-type plasminogen activator gene in human brain gliomas. J Surg Oncol. (2000) ;74: (2):90–4. |
[40] | Urquiaga I , Ávila F , Echeverria G , Perez D , Trejo S , Leighton F . A Chilean Berry Concentrate Protects against Postprandial Oxidative Stress and Increases Plasma Antioxidant Activity in Healthy Humans. Oxid Med Cell Longev. (2017) ;2017: :8361493. |
[41] | Cirak B , Inci S , Palaoglu S , Bertan V . Lipid peroxidation in cerebral tumors. Clin Chim Acta. (2003) ;327: (1):103–7. |
[42] | Alhosin M , León-González AJ , Dandache I , Lelay A , Radish SK , Kevers C , Pincemail J , Fornecker LM , Mauvieux L , Herbrecht R , Schini-Kerth VB . Bilberry extract (Antho 50) selectively induces redox-sensitive caspase 3-related apoptosis in chronic lymphocytic leukemia cells by targeting the Bcl-2/Bad pathway. Sci Rep. (2015) ;5: :8996. |
[43] | Cui HX , Chen JH , Li JW , Cheng FR , Yuan K . Protection of Anthocyanin from Myrica rubra against Cerebral Ischemia-Reperfusion Injury via Modulation of the TLR4/NF-κB and NLRP3 Pathways. Mol. (2018) ;23: (7):1788. |
[44] | Shin DY , Lee WS , Lu JN , Kang MH , Ryu CH , Kim GY , Kang HS , Shin SC , Choi YH . Induction of apoptosis in human colon cancer HCT-116 cells by anthocyanins through suppression of Akt and activation of p38-MAPK. Int J Oncol. (2009) ;35: :1499–504. |
[45] | Devi PS , Kumar MS , Das SM . Evaluation of antiproliferative activity of red sorghum bran anthocyanin on a human breast cancer cell line (mcf-7). Int J Breast Cancer. 2011:891481. |
[46] | Bunea A , Rugină D , Scont̆a Z , Pop RM , Pintea A , Socaciu C , Tăbăran F , Grooteart C , Strujis K , VanCamp J . Anthocyanin determination in blueberry extracts from various cultivars and their antiproliferative and apoptotic properties in B16-F10 metastatic murine melanoma cells. Phytochem. (2013) ;95: :436–44. |
[47] | Liu W , Xu J , Wu S , Liu Y , Yu X , Chen J , Tang X , Wang Z , Zhu X , Li X . Selective anti-proliferation of HER2-positive breast cancer cells by anthocyanins identified by high-throughput screening. PLoS One. (2013) ;8: (12):e81586–e81586. |



