Immune Parameters and COVID-19 Infection – Associations With Clinical Severity and Disease Prognosis
- 1Department of Pediatrics, Jessenius Faculty of Medicine, Comenius University in Bratislava, University Hospital in Martin, Martin, Slovakia
- 2Department of Pulmonology and Phthisiology, Jessenius Faculty of Medicine, Comenius University in Bratislava, University Hospital in Martin, Martin, Slovakia
- 3Department of Clinical Immunology and Allergology, University Hospital in Martin, Martin, Slovakia
- 4Department of Pediatrics, Faculty of Medicine, Children Faculty Hospital, P. J. Safarik University, Kosice, Slovakia
- 5Department of Pediatric Infectology, Faculty of Medicine, Children Faculty Hospital, P. J. Safarik University, Kosice, Slovakia
- 6Pneumo-Alergo Centre Ltd., Falck Healthcare Group, Bratislava, Slovakia
- 7Department of Pediatric Pulmonology and Phthisiology, Faculty of Medicine, National Institute of Children Diseases, Slovak Medical University, Bratislava, Slovakia
Severe acute respiratory syndrome caused by a novel 2019 coronavirus (SARS-CoV2) represents one of the most studied infectious diseases of today. The number of scientific reports and publications increases exponentially day by day. While the majority of infected subjects are asymptomatic or show mild symptoms, there is an important proportion of patients who requires hospitalization and, sometimes, intensive care. Immune response to novel coronavirus is complex, involves both innate and adaptive immunity, and is biphasic. Significant differences were observed when comparing severe and non-severe patients. Analysis of the reported results from clinical trials clearly show an involvement of specific cellular immunity (predominantly leucopenia, decreased counts of CD3+, CD4+, and CD8+ T lymphocytes, changes of T cell compartment) and the so-called cytokine storm, which is associated with worsening of symptoms and the promotion of lung damage. An interesting finding regarding eosinopenia that can have both diagnostic and prognostic value is reported by some authors. Examination of selected immune parameters could help to identify severe patients with the risk of unfavorable course of the disease, predict the prognosis and recognize improvement in the clinical status. Moreover, detailed analysis of the immune changes could help to select novel prospective therapeutic strategies.
Introduction – COVID-19 and the Immune System
The world is facing a global pandemic of severe acute respiratory syndrome caused by a novel 2019 coronavirus (SARS-CoV2). The diseases caused by this novel coronavirus was named COVID-19 (Coronavirus Disease 2019). The number of infected subjects increasing exponentially day by day is accompanied by an increasing number of patients in critical status and those who die. It is evident that the immune system plays a crucial role in the response to SARS-CoV2 with significant differences among severe and non-severe patients (Shi et al., 2020). It is suggested that evaluating the selected immune parameters could help us to understand better the dynamics of immune system activation and probably to select the appropriate prognostic markers with respect to disease outcomes and expected complications. Moreover, immune support treatment could be another possible step in the complex management of these patients. On the other hand, it is not clear whether the observed changes in immune parameters are direct consequence of COVID-19 or are predisposing factors for this infection and its severe course. Several questions regarding the immune response and its dynamics remain unresolved and the ongoing studies should bring the answers.
Immune response in SARS-CoV1, MERS and other viral pneumonias similar to SARS-CoV2 were recently reviewed and summarized (Lin et al., 2020; Vardhana and Wolchok, 2020; Yi et al., 2020). However, a complex review of the immune changes in COVID-19 patients with respect to the clinical picture, complications and severity is not yet available. We aimed to summarize the most important changes in inflammatory markers and the selected immune laboratory parameters, which were extracted from the available published literature.
Immune Response to COVID-19
Immune response to COVID-19 infections consists of two phases: In the beginning, during the phase of incubation and non-severe stages, a specific adaptive immune response is necessary to reach the control over virus proliferation, to eliminate the virus, and to prevent disease progression. Later, in certain patients (with comorbidities, of older age, and probably with specific genetic background) the cytokine release syndrome (so-called cytokine storm) promotes the progression to severe status and organ damage (Shi et al., 2020). Therefore, the host immune system is on one hand essential for the resolution of COVID-19 infection, but on the other can serve as a crucial player in the pathogenesis of the major clinical complications of the disease (Favalli et al., 2020). Several studies have shown various changes in selected immune laboratory parameters during the COVID-19 infection by comparing infected and healthy, asymptomatic and symptomatic, and severe and non-severe patients (Table 1).
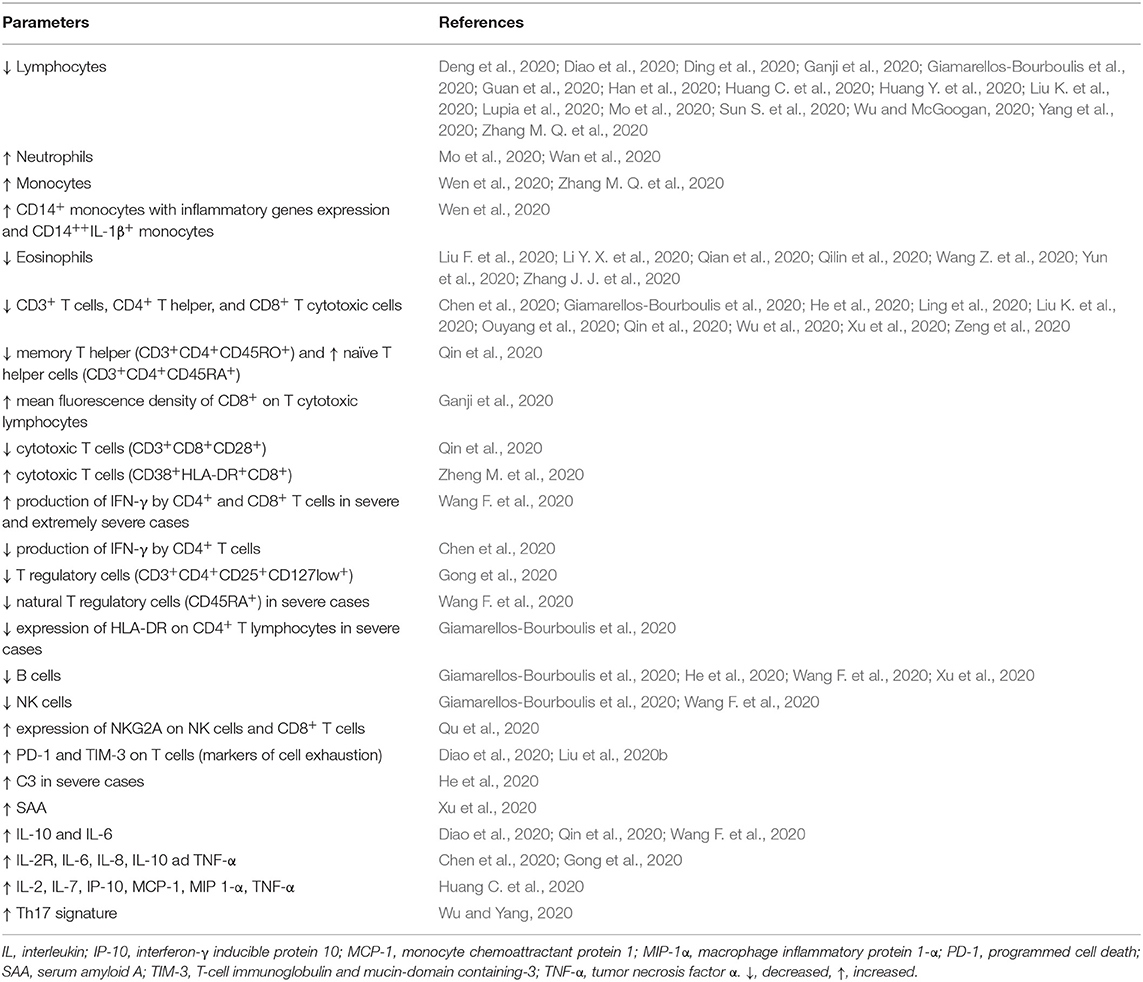
Table 1. Summary of the most important findings concerning immune parameters in patients with COVID-19.
Inflammatory Response in COVID-19
COVID-19 activates the immune system via different receptors; among them, Toll-like receptors (TLR-3, 4, and 7) are some of the most crucial. The binding of COVID-19 to TLRs activates the formation of active IL-1β and IL-6. These two cytokines are the central proinflammatory molecules causing the systemic clinical symptoms (malaise, fever, myalgia, etc.) and also leading to inflammation of the lungs (Conti et al., 2020).
Increased C-reactive protein (CRP) and high-sensitivity CRP is reported in the majority of COVID-19 patients. The highest values are usually observed in the most severe cases. The elevation of other inflammatory cytokines and chemokines, IL-2R, IL-6, IL-8, IL-10, and TNF-α, was found especially in severe cases compared to mildly affected individuals (Chen et al., 2020; Gong et al., 2020). Severe COVID-19 cases yielded higher concentrations of inflammatory markers compared to moderate patients (Chen et al., 2020). A cytokine profile similar to that of patients with secondary hemophagocytic lymphohistiocytosis, including increased IL-2, IL-7, interferon-γ-inducible protein 10, monocyte chemoattractant protein 1, macrophage inflammatory protein 1-α, and TNF-α was reported by Huang C. et al. (2020). Among non-survivors, an elevation of serum ferritin and IL-6 was one of the significant predictors (Ruan et al., 2020). Therefore, it could be suggested that, besides antiviral treatment, a balanced immunosuppression (e.g., by using the inhibitors of selected cytokines – IL-6, IL-1) could be another effective approach to reduce and modulate viral induced hyperinflammation (storm) and thus to prevent severe and irreversible organ damage that contributes to the relatively high mortality of COVID-19 patients (Conti et al., 2020; Mehta et al., 2020). Moreover, based on the previous experience with SARS, the cytokine storm is strongly associated with pulmonary inflammation and excessive lung damage. Similar to MERS-CoV and SARS-CoV-1, inflammation in COVID-19 patients is also characterized by Th17 cytokine signature. Therefore, a selective JAK inhibitor (e.g., fedratinib) could present an option for treatment of these patients with severe course by affecting the production of Th17-cytokines (Wu et al., 2020). On the other hand, several authors showed that the early stage of inflammatory response to SARS-CoV-2 is characterized by weakened interferon production from the infected cells which can result in the progression of the infection (Chu et al., 2020). In contrast with the other pro-inflammatory cytokines that should be therapeutically decreased and blocked, application of interferons (e.g., interferon lambda) could present another therapeutic strategy, especially in the early stages of COVID-19 (O'Brien et al., 2020).
White Blood Cell Counts and COVID-19
Substantial numbers of clinical studies and observations have reported lymphopenia in a significant proportion of patients with confirmed COVID-19 infection (Chen et al., 2020; Ding et al., 2020; Guan et al., 2020; Han et al., 2020; Huang C. et al., 2020; Lin et al., 2020; Liu K. et al., 2020; Lupia et al., 2020; Mo et al., 2020; Sun S. et al., 2020; Wu and McGoogan, 2020; Yang et al., 2020; Zhang M. Q. et al., 2020). Lymphopenia could be considered as a signature for severe COVID-19 infection and pneumonia (Bermejo-Martin et al., 2020). The highest proportion of lymphopenic patients (83.2%) was reported by Guan et al. (2020). In a group of 10 patients with COVID-19 pneumonia, the authors investigated the differential white blood cells counts. COVID-19 patients had lower absolute numbers of lymphocytes and eosinophils compared to the other 30 patients with non-COVID-19 pneumonia (Li Y. X. et al., 2020). Comparing mild and severe cases of COVID-19, a more profound decline in the absolute number of lymphocytes was particularly observed in severe cases and critically ill patients (Chen et al., 2020; Peng et al., 2020; Wan et al., 2020). In contrast, severe cases were frequently characterized by neutrophilia (Mo et al., 2020; Wan et al., 2020). Monocytosis was also observed sporadically in this group of patients (Zhang M. Q. et al., 2020). During the early recovery stage of COVID-19, an increase of CD14+ monocytes with inflammatory gene expression as well as abundance of CD14++IL-1β+ monocytes were recently described (Wen et al., 2020). Patients with abnormal lung imaging findings had lower lymphocytes compared to patients without lung involvement (Zhang X. et al., 2020). Lymphopenia was also seen in a small group of COVID-19 patients co-infected with influenza. Interestingly, the course of the disease and laboratory and imaging findings were similar to those with COVID-19 only (Ding et al., 2020). The proportion of lymphocytes was significantly lower in the elderly group compared to the young and middle-aged groups (Liu K. et al., 2020). Compared to adult patients, infants and young children usually have a mild course of disease without reductions in leukocyte or lymphocyte counts (Zhou et al., 2020a,b). However, up to now, only a few reports containing detailed data about children infected with this novel virus are available in the literature, so conclusions cannot be drawn. It is evident, that in children the immune response to COVID-19 is different compared to the adults and majority of the children have mild symptoms or asymptomatic course. This could be explained by higher capacity to produce natural antibodies with broad reactivity, presence of physiologic lymphocytosis and differences in lymphocyte compartment, virus-to-virus interactions in the airways (simultaneous presence of other respiratory viruses in the airway mucosa), differences in the expression of ACE2, different inflammatory response in children and many others aspects (Brodin, 2020; Carsetti et al., 2020). On the other hand, recently described COVID-19-associated pediatric multi-system inflammatory syndrome shows several differences to the course of COVID-19 in adults and is highly specific for children (Deza Leon et al., 2020).
Another interesting finding reported by several author groups is the eosinopenia associated with COVID-19 infections (Jesenak et al., 2020). Recent findings suggest that eosinophils have important antiviral properties (Flores-Torres et al., 2019). Some eosinophil-derived granular proteins (e.g., eosinophil-derived neurotoxin, eosinophil cationic protein) show antiviral activity against single-stranded RNA viruses. Moreover, eosinophils are able to produce nitric oxide and can induce CD8+ T cell proliferation and activation as a response to virus- or viral-peptide exposure. Eosinophils can also support viral clearance (Jesenak and Schwarze, 2019). Taking into account all these facts, the reported eosinopenia in COVID-19 patients is of special interest (Du et al., 2020; Li Y. X. et al., 2020; Qian et al., 2020; Zhang J. J. et al., 2020). Li Y. X. et al. (2020) compared 10 COVID-19 patients with 30 patients affected by other viral pneumonia. They found that leukopenia, lymphocytopenia, and eosinopenia were more common in COVID-19 patients compared to non-COVID-19 subjects. Detailed investigations of 140 hospitalized patients suggested that eosinopenia with lymphopenia may be a potential indicator for COVID-19 with both diagnostic and prognostic value (Zhang J. J. et al., 2020). Combination of eosinopenia together with elevated high-sensitivity CRP could effectively triage suspected patients with COVID-19 from the other patients with fever (Qilin et al., 2020). Eosinopenia indicated poor prognosis also in another study (Du et al., 2020). The increase in eosinophils observed during treatment from the initially low levels could be a positive indicator of clinical improvement (Liu F. et al., 2020; Sun S. et al., 2020). On the other hand, pooled analysis suggested that eosinopenia may not be associated with unfavorable progression of COVID-19 (Lippi and Henry, 2020). The role of eosinophils in COVID-19 is still discussed. It seems that observed eosinopenia has more diagnostic and eventually prognostic value then real participation on the COVID-19 pathology (Lindsley et al., 2020; Qian et al., 2020). Whether it is the consequence of the block in eosinopoiesis, result of the decreased release of eosinophils from bone marrow or IFN-induced apoptosis remains to be answered in further studies.
Specific Cellular Immunity Parameters
Based on the current knowledge that T lymphocytes play a central role in the protection against coronaviruses, it is of special interest to analyze the possible relationship of lymphocytes and their subpopulations relative to the clinical course and complications of COVID-19. T cells are mostly affected by COVID-19 (Chen et al., 2020; Ouyang et al., 2020; Qin et al., 2020; Sun D. W. et al., 2020; Sun S. et al., 2020; Wu and McGoogan, 2020). A study in 752 patients analyzed the subpopulations of lymphocytes (CD3+, CD4+, CD8+). They compared the findings in COVID-19 patients with the normal reference values of the Chinese population. CD3+ lymphocytes below 900 cells/mm3, CD4+ cells below 500 cells/mm3, and CD8+ lymphocytes below 300 cells/mm3 were considered to select the subjects at higher risk of COVID-19 infection. Significant differences in the numbers of CD4+ and CD8+ T lymphocytes between mild and severe/critical COVID-19 cases were described (Chen et al., 2020). No differences between B-cells and NK cell numbers were usually found (Sun D. W. et al., 2020; Sun S. et al., 2020; Zeng et al., 2020), however, recent studies reported also the decline in B cells numbers (Xu et al., 2020). Another authors' group reported that the non-intensive care unit patients with total T cells, CD4+ and CD8+ cell counts lower than 800, 400, and 300/μL, respectively, require attention and intervention even in immediate absence of more severe symptoms due to a high risk for further deterioration (Diao et al., 2020). Moreover, lower counts of T lymphocytes and their subsets (total CD3+ <200/μL, CD4+ <100/μL, and CD8+ <100/μL) were significantly associated with higher risk of in-hospital death due to COVID-19. The warning values to predict in-hospital death of lymphocytes, CD3+, CD4+, CD8+, and CD19+ cells were 559, 235, 104, 85, and 82, respectively (Xu et al., 2020). Similar results found also He et al. (2020). In several studies, a decline in CD8+ was more frequently observed in COVID-19 patients than a decline in CD4+. Decreased numbers of CD8+ T lymphocytes were also reported by other groups, especially in connection with the presence of lung involvement and pneumonia development (Liu et al., 2020a). CD8+ T cytotoxic cells can contribute to the elimination of virus by production of many biologically active molecules, such as perforins, granzymes and interferons. Therefore, the decline number of CD8+ T cells along with the dysfunction could significantly contribute to the severe course of COVID-19 and loss of control over virus production. An interesting observation brought a study of Ganji et al. (2020). While no differences in CD4:CD8 ratio, CD4+ and CD8+ T cell numbers and CD4 mean fluorescence intensity did not differ between COVID-19 patients and healthy individuals, mean fluorescence intensity of CD8 expression on T cytotoxic cells increased significantly in COVID-19 infected patients. This could the sign of hyperactivation of T cytotoxic cells as a response to SARS-CoV2 infection (Ganji et al., 2020). In contrast, a series of other studies found more significant differences in CD4+ T helper cell numbers (Qin et al., 2020). Moreover, decreased production of IFN-γ by CD4+ T cells were observed in the severe cases (Chen et al., 2020). A detailed analysis of T cell compartment showed the analysis of the laboratory results of 452 COVID-19 patients, among which 286 were diagnosed with severe infection. Severe cases had significantly decreased T cells, especially CD4+ T helper cells. The percentage of naïve helper T cells (CD3+CD4+CD45RA+) increased, and memory helper T cells (CD3+CD4+CD45RO+) decreased in severe cases. Similarly, CD28+-positive cytotoxic T cells (CD3+CD8+CD28+) were decreased in severe cases. Moreover, COVID-19 patients had lower level of regulatory T cells (CD3+CD4+CD25+CD127low+), especially in severe cases. No differences were found for activated helper or suppressor T cells (expressing HLA-DR+). Interestingly, compared to the changes in absolute numbers of T cells and NK cells, their functions (indicated by PMA/Ionomycin-stimulated IFN-γ cells) remained within the normal range, with no differences between severe and non-severe cases (Qin et al., 2020). The percentage of natural T regulatory cells (CD45RA+ Treg) was significantly decreased in severe patients, especially in those with extremely severe illness (Wang F. et al., 2020). Regulatory T cells are crucial in the maintenance of the immune homeostasis in various T cells subpopulation and their activity and they determine the effective and balanced immune response. Therefore, the decreased number and function of T regulatory cells are of special interest as a potential therapeutic target. The same results regarding the decline in T cells and CD4+ T helper cells was also reported in the study by Wu et al. (2020). It was suggested that the absolute number of CD4+ T lymphocytes may predict the duration of viral RNA in the stools of infected patients: The lower the CD4+ count, the longer the duration of viral RNA persistence in the stool (Ling et al., 2020). A pathological autopsy report of three COVID-19 patients who died due to pneumonia showed that the alveoli were infiltrated mainly with macrophages and monocytes with minimal infiltrating lymphocytes, eosinophils and neutrophils. Most of the infiltrated lymphocytes were CD4+ T cells (Yao et al., 2020). Another important immunopathology that could contribute to the immune response to COVID-19 is the Th17 immune response activated in the environment of overproduction (Hotez et al., 2020). The response of immune system and susceptibility to different infections is determined by many factors, among them HLA haplotypes play an essential role. It could be suggested that the specific cellular response would be modified by specific HLA haplotypes, which can affect the characteristics of the developing anti-viral immunity and its efficacy in the achievement of control over viral infections (Shi et al., 2020). T-cell activation is probably compromised by infected antigen presenting cells (Shin et al., 2019). The observed lymphopenia could have various explanations. It could be the results of virus induced apoptosis or so-called pyroptosis (e.g., cell death induced by IL-1β). Moreover, also the initial viral load could probably modify the final immune response.
Only a few studies have also analyzed the functional changes of lymphocytes. As referred to above, in a study of Qin et al. (2020), despite significantly decreased lymphocyte counts, their function was not diminished. The kinetics of immune response in relation to clinical and virological features in one patient with mild-to-moderate COVID-19 was recently published in detail (Thevarajan et al., 2020). The authors described an increase in antibody-secreting cells, follicular helper T cells, activated CD4+ and CD8+ T cells, and immunoglobulin IgG- and IgM-binding COVID-19 in the blood before symptomatic recovery. A rapid increase in the co-expression of HLA-DR and CD38 on CD8+ T cells (CD38+HLA-DR+CD8+) was detected before clinical status improvement. This phenotype of cells produced large amounts of potent antimicrobial agents (granzymes A and B, perforin; Thevarajan et al., 2020). In severe patients with respiratory failure, expression of HLA-DR on CD4+ T cells was markedly decreased. Moreover, SARS-CoV-2 patient plasma inhibited HLA-DR expression (Giamarellos-Bourboulis et al., 2020). All the cited studies are confirming that the associated cytokine storm negatively affects the activation and effective functions of the immune cells. A selective inhibition of particular cytokine could have profound effect on the restoring the immune functions and improving the clinical status.
Other possible consequence of insufficient immune control over SARS-CoV-2 infection could be also the anergy and exhaustion of immune cells, which can be measured by various surface markers. NKG2A (C-type lectin receptor with inhibitory effects) expression on NK cells and CD8+ cells affects their activation. In COVID-19 patients, increased expression of NKG2A on NK cells and CD8+ led to functional exhaustion of both cell populations. During the convalescent period, the total numbers of NK cells and CD8+ T lymphocytes increased, accompanied by decreased expression of NKG2A. These findings suggest that the downregulation of NKG2A expression may correlate with disease control and improvement in clinical status (Zhang X. et al., 2020). Whether this is a direct effect of COVID-19 remains unclear; however, it is clear that protective antiviral immunity is strongly affected in these patients. This could be the result of the direct effect of coronavirus, or it could be the result of the associated cytokine storm. Targeting NKG2A may prevent the functional exhaustion of antiviral immune cellular protection and contribute to virus elimination. Other study showed that besides dramatically decreased numbers of total T cells, CD4+ and CD8+ T cell subsets, T cells from COVID-19 patients had significantly higher levels of the exhausted marker PD-1 (programmed cell death 1; CD279) compared to healthy controls. Moreover, gradual increase of PD-1 and another co-inhibitory receptor TIM-3 (T-cell immunoglobulin and mucin-domain containing-3; CD366) expression on the T cells was accompanying the progression from the prodromal to severe symptomatic stages (Diao et al., 2020). This suggests that the exhaustion of T cells (so-called immunoparalysis) could contribute to the ineffective immune control over virus replication and to the progression of the diseases into severe stages. Therefore, therapeutic strategies blocking PD-1/PD-1L and TIM3 pathways could have a significant impact on the outcome of COVID-19 patients with low-to-medium expression of these markers. On the other hand, the patients with high expression of CD366 (TIM-3) would have worse prognosis with limited effect of immune interventions (Chiappelli et al., 2020). Similar results published also another group (Liu et al., 2020a). Besides the measurement of the surface markers on the cells, interesting results brought the study evaluating the expression of different genes during the COVID-19 progression and subsequent treatment. During the disease progression, a down-regulation of the genes involved in Th17 cell differentiation, cytokine-mediated signaling and T cell activation was evident. After the treatment in severe cases, MAP2K7 and SOS1 (both involved in the T-cell activation and signaling) were upregulated (Ouyang et al., 2020).
Immunoglobulins and Complement
Surprisingly, immunoglobulin values and complement components are only occasionally reported in the published cohorts, probably due to normal observed levels. Qin et al. (2020) did not see any changes of immunoglobulin isotypes (IgG, IgA, IgM) and complement proteins (C3, C4) in COVID-19 patients. No differences were found when comparing mild and severe patients, with the exception of IgM, which was lower in severe cases (Qin et al., 2020). A certain time before clinical improvement, and increases in antibody-producing cells and specific immunoglobulins (IgM, IgG) binding COVID-19 could be observed (Thevarajan et al., 2020). In severe cases, increased level of C3 can probably represents a possible regulatory factor in the context of systemic inflammation (He et al., 2020).
Immune Parameters as Prognostic Factors
In a study by Qu et al. (2020), positive correlations between platelet-to-lymphocyte ratio (PLR), peak platelet numbers and severity of the disease were confirmed. The average hospitalization days of patients with platelet peaks were longer than those without; moreover, the patients with platelet peaks were older compared to those without. It was confirmed that the greater the difference between the PLR at admission and during treatment, the greater possibility of severe pneumonia. The authors suggested that the platelet peaks could be related to the cytokine storm described in COVID-19 infection (Qu et al., 2020). The early clinical and laboratory findings of COVID-19 pneumonia are low-to-midgrade fever, dry cough, and fatigue, with normal white blood cell count, reduced lymphocyte count and elevated CRP (Han et al., 2020). Eosinopenia could represent another prognostic factor (Du et al., 2020) and the increase of eosinophils may serve as a positive predictive factor of clinical improvement (Liu F. et al., 2020; Sun S. et al., 2020). Detailed analysis of the immune parameters' changes identified certain possible prognostic factors. Several authors identify a degree of T-cell decline to be a negative predictive factor for disease course (Diao et al., 2020; He et al., 2020; Xu et al., 2020). The changes of the expression of inhibiting markers (e.g., NKG2A, TIM-3) on the cell surface could serve as another possible prognostic factor (Chiappelli et al., 2020; Liu et al., 2020a; Zheng M. et al., 2020). It should be concluded, that early recognition of the immune phenotype associated with disease progression could help to identify the most severe and risky patients, with the modification of the treatment procedure.
Immuno-Interventional Approach in COVID-19 Treatment
It is evident that the treatment approach should consist of the combination of several drugs potentially affecting the particular components of COVID-19 infections (Table 2). The majority of the protocols include the application of antiviral drugs (mainly oseltamivir, ganciclovir, lipinavir/ritonavir, umifenovir, fevipiravir, and experimental drug remdesivir), anti-malarial medicaments (chloroquine phosphate, hydrochloroquine; Cunningham et al., 2020) or azalides (azithromycin). Besides these approaches, cytokine-targeted monoclonal antibodies (anti-IL-1 – anakinra, cakakinumab or anti-IL-6 – tocilizumab) and JAK inhibitors (e.g., fedrotinib) are also considered as potential therapies (Conti et al., 2020; Thevarajan et al., 2020; Wu et al., 2020). These treatments are especially for moderate and severe cases requiring hospitalization.
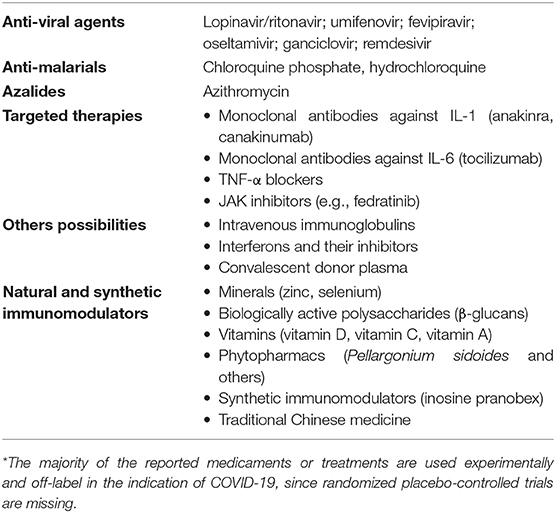
Table 2. Current possibilities and approaches in the treatment* of COVID-19 (adapted and modified from Cunningham et al., 2020; Jayawardena et al., 2020; Ye et al., 2020).
However, are there any possibilities that may support the immune system in mild cases? Despite the lack of clinical data, certain studies and experimental data suggested the potential role of zinc in coronavirus infections. Recent review summarized the possible useful mode of actions of zinc in the management of COVID-19 (Skalny et al., 2020). Zinc inhibits RNA polymerase activity and blocks the virus replication in vitro (Te Velthuis et al., 2010). Zinc ions also show an ability to inhibit SARS-COV protease (Lee et al., 2007). Moreover, the general support of anti-viral immunity (e.g., production of interferons) and complex anti-inflammatory activity by inhibiting NF-κB signaling could be beneficial in the context of known effects of SARS-CoV2 in the organisms (Skalny et al., 2020). Zinc could improve the efficacy of chloroquine and hydroxychloroquine which are currently used in COVID-19 infection (Shitti and Afolami, 2020).
Biologically active polysaccharides (e.g., β-glucans) represent a highly studied group of natural immunomodulators with pluripotent biological activities. Certain molecules are able to attenuate inflammatory cytokine release and prevent lung injury in animal models (Bedirli et al., 2007; Cao et al., 2018) and restore the cytokine imbalance by promoting the secretion of anti-inflammatory compounds (Chen et al., 2013). Another mode of action could be the support of NK cell functions and modulatory effect on T cells (Bobovak et al., 2010; Bergendiova et al., 2011; Jesenak et al., 2013). A recent study confirmed the role of pleuran (β-glucan isolated from Pleurotus ostreatus) in the treatment of acute herpes simplex type 1 infection (Urbancikova et al., 2020). Using in vitro model of lung injury, lentinan (β-glucan from Lentinus edodes) reduced cytokine-induced NF-κB activation in human alveolar epithelial cells and attenuated pro-inflammatory cytokine production (TNF-α, IL-2, 6, 8, 22) as well as TGF-β and IL-10 (Murphy et al., 2020). A possible role of α-glucans has also been suggested recently (Di Pierro et al., 2020).
A special interest is focused on the possible role of vitamin D and its deficiency in the risk for COVID-19 and its severe course. Vitamin D has pluripotent modulatory activities on both innate and specific immunity. Its deficiency is a risk factor for exaggerated and persistent inflammation (Grant et al., 2020). Moreover, its deficiency increases the risk of severe course of COVID-19 and could at least partly explain the geographic variations in the case fatality rate of COVID-19 (Garg et al., 2020; Marik et al., 2020). Taking into consideration all of these facts, supplementation of vitamin D in an appropriate dose could have both preventive and therapeutic effect in COVID-19 (Ilie et al., 2020; Silberstein, 2020). Another vitamin with potential role in the management of COVID-19 could be vitamin C. It was shown that vitamin C is beneficial to critical care management (Nabzdyk and Bittner, 2018) and shortened the intensive care unit stay with the reduction in the mortality rate (Marik et al., 2017). Vitamin C reduced the mortality rate in the patients with sepsis-related ARDS (Kim et al., 2018). Therefore, several authors suggested the potential role of high-dose vitamin C in the treatment and prevention of COVID-19 along with the ongoing clinical trials (Carr, 2020).
Certain phytotherapeuticals (e.g., Pelargonium sidoides extract) have also yielded a positive effect on the proliferation of coronaviruses (Michaelis et al., 2011). Several authors have also suggested the potential role of selected Chinese herbal medicines (Li R. et al., 2020; Zhang D. H. et al., 2020).
Synthetic immunomodulators directed at a specific cellular immunity could represent another option. Some of them (e.g., inosine pranobex) showed activity against a broad spectrum of respiratory viruses (Beran et al., 2016).
Conclusions and Perspectives
The novel coronavirus mainly acts on lymphocytes, especially T cells. Analysis of lymphocyte subsets could be helpful in the early screening of the potential critical course of COVID-19 disease. The consumption of CD4+ and CD8+ cells, increased concentration of a broad spectrum of proinflammatory cytokines and chemokines, and decreased T regulatory cells could contribute to the excessive inflammatory response (cytokine storm, cytokine release syndrome) with a loss of control over the damaging immune response and the promotion of tissue damage (e.g., in the lungs). Besides other reported laboratory anomalies associated with the unfavorable progression of COVID-19 (e.g., decreased albumin, increased lactate dehydrogenase, alanine aminotransferase, aspartate aminotransferase, bilirubin, creatinine, cardiac troponin, D-dimer, procalcitonin, and CRP; Lippi and Plebani, 2020), special attention has to be paid to the parameters of the immune system. Decreased lymphocyte and eosinophil counts, with deterioration of other parameters of cellular immunity, should be carefully assessed and regularly checked in COVID-19 patients to monitor the course of the disease and predict worsening of the symptoms.
Author Contributions
MJ was involved analysis and interpretation of the data, literature search, and in drafting and revising the manuscript. MB was involved in analysis and interpretation of the data discussed in the manuscript, and in revising the manuscript. IU was involved in the literature search and in drafting and revising the manuscript. ZR was involved in the literature search, critical analysis of the data, and in revising the manuscript. JV was involved in revising the manuscript. AB was involved in drafting and revising the manuscript. RO was involved in analysis and interpretation of the data and in revising the manuscript. PB was involved in analysis of the data and in revising the manuscript. All the authors have approved the final version of the manuscript.
Funding
This study was co-funded by the project VEGA 1/0310/18.
Conflict of Interest
ZR was employed by Pneumo-Alergo Centre Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Bedirli, A., Kerem, M., Pasaoglu, H., Akyurek, N., Tezcaner, T., Elbeg, S., et al. (2007). Beta-glucan attenuates inflammatory cytokine release and prevents acute lung injury in an experimental model of sepsis. Shock 27, 397–401. doi: 10.1097/01.shk.0000245030.24235.f1
Beran, J., Salapova, E., and Spajdel, M. (2016). Inosine pranobex is safe and effective for the treatment of subjects with confirmed acute respiratory viral infections: analysis and subgroup analysis from a phase 4, randomized, placebo-controlled, double-blind study. BMC Infect. Dis. 16:648. doi: 10.1186/s12879-016-1965-5
Bergendiova, K., Tibenska, E., and Majtan, J. (2011). Pleuran (β-glucan from pleurotus ostreatus) supplementation, cellular immune response and respiratory tract infections in athletes. Eur. J. Appl. Physiol. 111, 2033–2040. doi: 10.1007/s00421-011-1837-z
Bermejo-Martin, J. F., Almansa, R., Menendez, R., Mendez, R., Kelvin, D. J., and Torres, A. (2020). Lymphopenic community acquired pneumonia as signature of severe COVID-19 infection. J. Infect. 80, e23–e24. doi: 10.1016/j.jinf.2020.02.029
Bobovak, M., Kuniakova, R., Gabriz, J., and Majtan, J. (2010). Effect of pleuran (β-glucan from Pleurotus ostreatus) supplementation on cellular immune response after intensive exercise in elite athletes. Appl. Physiol. Nutr. Metab. 35, 755–762. doi: 10.1139/H10-070
Brodin, P. (2020). Why is COVID-19 so mild in children? Acta Paediatr. 109, 1082–1083. doi: 10.1111/apa.15271
Cao, Y., Sun, Y., Zou, S., Duan, B., Sun, M., and Xu, X. (2018). Yeast β-glucan suppresses the chronic inflammation and improves the microenvironment in adipose tissue of ob/ob mice. J. Agric. Food. Chem. 66, 621–629. doi: 10.1021/acs.jafc.7b04921
Carr, A. C. (2020). A new clinical trial to test high-dose vitamin C in patients with COVID-19. Crit. Care 24:133. doi: 10.1186/s13054-020-02651-4
Carsetti, R., Quintarelli, C., Quinti, I., Mortari, E. P., Zumla, A., Ippolito, G., et al. (2020). The immune system of children: the key to understanding SARS-CoV-2 susceptibility? Lancet 4, P414–P416. doi: 10.1016/S2352-4642(20)30135-8
Chen, G., Wu, D., Gue, W., Cao, Y., Huang, D., Wang, H., et al. (2020). Clinical and immunological features in severe and moderate coronavirus disease 2019. J. Clin. Investig. 130, 2620–2629. doi: 10.1172/JCI137244
Chen, Y., Dong, L., Weng, D., Liu, F., Song, L., Li, C., et al. (2013). 1,3-β-glucan affects the balance of Th1/Th2 cytokines by promoting secretion of anti-inflammatory cytokines in vitro. Mol. Med. Rep. 8, 708–712. doi: 10.3892/mmr.2013.1553
Chiappelli, F., Khakshooy, A., and Greenberg, G. (2020). COViD-19 immunopathology & immunotherapy. Bioinformation 16, 219–222. doi: 10.6026/97320630016222
Chu, H., Chan, J. F. W., Wang, Y., Yuen, T. T. T., Chai, Y., Hou, Y., et al. (2020). Comparative replication and immune activation profiles of SARS-COV-2 and SARS-CoV in human lungs: an ex vivo study with implications for the pathogenesis of COVID-19. Clin. Infect. Dis. 9:ciaa410. doi: 10.1093/cid/ciaa410
Conti, P., Ronconi, G., Craffa, A., Gallenga, C. E., Ross, R., Frydas, I., et al. (2020). Induction of pro-inflammatory cytokines (IL-1 and IL-6) and ling inflammation by COVID-19: anti-inflammatory strategies. J. Biol. Regul. Homeost. Agents. 34:2. doi: 10.23812/CONTI-E
Cunningham, A. C., Goh, H. P., and Koh, D. (2020). Treatment of COVID-19: old tricks for new challenges. Crit. Care 24:91. doi: 10.1186/s13054-020-2818-6
Deng, Y., Liu, W., Liu, K., Fang, Y. Y., Shang, J., Zhou, L., et al. (2020). Clinical characteristics of fatal and recovered cases of coronavirus disease 2019 (COVID-19) in Wuhan, China: a retrospective study. Chin. Med. J. 133, 1261–1267. doi: 10.1097/CM9.0000000000000824
Deza Leon, M. P., Redzepi, A., McGrath, E., Abdel-Haq, N., Shawaqfeh, A., Sethuraman, U., et al. (2020). COVID-19 associated pediatric multi-system inflammatory syndrome. J. Pediatr. Infect. Dis. Soc. 9:piaa061. doi: 10.1093/jpids/piaa061
Di Pierro, F., Bertuccioli, A., and Cavecchia, I. (2020). Possible therapeutic role of a highly standardized mixture of active compounds derived from cultured Lentinula edodes myceli (AHCC) in patients infected with 2019 novel coronavirus. Minerv. Gastroenterol. Dietolog. 66, 172–176. doi: 10.23736/S1121-421X.20.02697-5
Diao, B., Wang, C., Tan, Y., Chen, X., Liu, Y., Ning, L., et al. (2020). Reduction and functional exhaustion of T cells in patients with coronavirus diseases 2019 (COVID-19). Front. Immunol. 11:827. doi: 10.3389/fimmu.2020.00827
Ding, Q., Lu, P., Fan, Y., Xia, Y., and Liu, M. (2020). The clinical characteristics of pneumonia patients coinfected with 2019 novel coronavirus and influenza virus in Wuhan, China. J. Med. Virol. doi: 10.1002/jmv.25781. [Epub ahead of print].
Du, Y., Tu, L., Zhu, P., Mu, M., Wang, R., Yang, P., et al. (2020). Clinical features of 85 fatal cases of COVID-19 from Wuhan: a retrospective observational study. Am. J. Respir. Crit. Care Med. 201, 1372–1379. doi: 10.1164/rccm.202003-0543OC
Favalli, E. G., Ingegnoli, F., De Lucia, O., Cincinelli, G., Cimaz, R., and Caporali, R. (2020). COVID-19 infection and rheumatoid arthritis: faraway, so close! Autoimmun. Rev. 19:102523. doi: 10.1016/j.autrev.2020.102523
Flores-Torres, A. S., Salinas-Carmona, M. C., Salinas, E., and Rosas-Taraco, A. G. (2019). Eosinophils and respiratory viruses. Viral. Immunol. 32, 198–207. doi: 10.1089/vim.2018.0150
Ganji, A., Farahani, I., Khansarinejad, B., Ghazavi, A., and Mosayebi, G. (2020). Increased expression of CD8 marker on T-cells in COVID-19 patients. Blood Cells Mol. 83:102437. doi: 10.1016/j.bcmd.2020.102437
Garg, M., Al-Ani, A., Mitchell, H., Hendy, P., and Christensen, B. (2020). Editorial: low population mortality from COVID-19 in countries of latitude 35 degrees North – supports vitamin D as a factor determining severity. Aliment. Pharmacol. Ther. 51, 1438–1439. doi: 10.1111/apt.15796
Giamarellos-Bourboulis, E. J., Netea, M. G., Rovina, N., Akinosoglou, K., Antoniadou, A., Antonakos, N., et al. (2020). Complex immune dysregulation in COVID-19 patients with severe respiratory failure. Cell Host Microbe. 27, 992–1000. doi: 10.1016/j.chom.2020.04.009
Gong, J., Dong, H., Xia, Q., Huang, Z., Wang, D., Zhao, Y., et al. (2020). Correlation analysis between diseases severity and inflammation related parameters in patients with COVID-19 pneumonia. Cell Host Microbe. 27, 992–1000.
Grant, W. B., Lahore, H., McDonnell, S. L., Baggerly, C. A., French, C. B., Aliano, J. L., et al. (2020). Evidence that vitamin D supplementation could reduce risk of influenza and COVID-19 infections and deaths. Nutrients 12:988. doi: 10.3390/nu12040988
Guan, W., Ni, Z., Hu, Y., Liang, W., Ou, C., He, J., et al. (2020). Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 382, 1708–1720. doi: 10.1056/NEJMoa2002032
Han, R., Huang, L., Jiang, H., Dong, J., Peng, H., and Zhang, D. (2020). Early clinical and CT manifestations of coronavirus disease 2019 (COVID-19) pneumonia. AJR. Am. J. Roentgenol. 17, 1–6. doi: 10.2214/AJR.20.22961
He, R., Lu, Z., Zhang, L., Fan, T., Xiong, R., Shen, X., et al. (2020). The clinical course and its correlated immune status in COVID-19 pneumonia. J. Clin. Virol. 127:104361. doi: 10.1016/j.jcv.2020.104361
Hotez, P. J., Bottazzi, M. E., and Corry, D. B. (2020). The potential role of Th17 immune responses in coronavirus immunopathology and vaccine-induced immune enhancement. Microbes Infect. 22, 165–167. doi: 10.1016/j.micinf.2020.04.005
Huang, C., Wang, Y., Li, X., Ren, L., Zhao, J., Hu, Y., et al. (2020). Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 395, 497–506. doi: 10.1016/S0140-6736(20)30183-5
Huang, Y., Tu, M., Wang, S., Chen, S., Zhou, W., Chen, D., et al. (2020). Clinical characteristics of laboratory confirmed positive cases of SARS-CoV-2 infection in Wuhan, China: A retrospective single center analysis. Trav. Med. Infect. Dis. 27:101606. doi: 10.106/j.tmaid.2020.101606
Ilie, P. C., Stefanescu, S., and Smith, L. (2020). The role of vitamin D in the prevention of coronavirus disease 2019 infection and mortality. Aging Clin. Exp. Res. 6, 1–4. doi: 10.1007/s40520-020-01570-8
Jayawardena, R., Sooriyaarachchi, P., Chourdakis, M., Jeewandara, C., and Ranasinghe, P. (2020). Enhancing immunity in viral infections, with special emphasis on COVID-19: a review. Diabetes Metab. Syndr. 14, 367–382. doi: 10.1016/j.dsx.2020.04.015
Jesenak, M., Banovcin, P., and Diamant, Z. (2020). COVID-19, chronic inflammatory respiratory diseases and eosinophils – observations from reported clinical case series. Allergy. doi: 10.1111/all.14353. [Epub ahead of print].
Jesenak, M., Majtan, J., Rennerova, Z., Kyselovic, J., Banovcin, P., and Hrubisko, M. (2013). Immunomodulatory effect of pleuran (β-glucan from Pleurotus ostreatus) in children with recurrent respiratory tract infections. Int. Immunopharmacol. 15, 395–399. doi: 10.1016/j.intimp.2012.11.020
Jesenak, M., and Schwarze, J. (2019). Lung eosinophils – a novel “virus sink” that is defective in asthma? Allergy. 74, 1832–1834. doi: 10.1111/all.13811
Kim, W. Y., Jo, E. J., Eom, J. S., Mok, J., Kim, M. H., Park, H. K., et al. (2018). Combined vitamin C, hydrocortisone, and thiamine therapy for patients with severe pneumonia who were admitted to the intensive care unit: propensity score-based analysis of a before-after cohort study. J. Crit. Care. 47, 211–218. doi: 10.1016/j.jcrc.2018.07.004
Lee, C. C., Kuo, C. J., Hsu, M. F., Liang, P. H., Fang, J. M., Shie, J. J., et al. (2007). Structural basis of mercury- and zinc-conjugated complexes as SARS-CoV 3C-like protease inhibitors. FEBS Lett. 581, 5454–5458. doi: 10.1016/j.febslet.2007.10.048
Li, R., Hou, Y., Huang, J., Pan, W., Ma, Q., Shi, Y., et al. (2020). Lianhuaqingwe exerts anti-viral and anti-inflammatory activity against novel coronavirus (SARS-COV-2). Pharmacol. Res. 156:104761. doi: 10.1016/j.phrs.2020.104761
Li, Y. X., Wu, W., Yang, T., Zhou, W., Fu, Y. M., Feng, Q. M., et al. (2020). Characteristics of peripheral blood leukocyte differential counts in patients with COVID-19. Zhoughua. Nei. Ke. Za. Zhi. 59:E003.3760. doi: 10.3760/cma.j.cn112138-20200221-00114
Lin, L., Lu, L., Cao, W., and Li, T. (2020). Hypothesis for potential pathogenesis of SARS-CoV-2 infection – a review of immune changes in patients with viral pneumonia. Emerg. Microb. Infect. 9, 727–732. doi: 10.1080/22221751.2020.1746199
Lindsley, A. W., Schwartz, J. T., and Rothenberg, M. E. (2020). Eosinophil responses during COVID-19 infections and coronavirus vaccination. J. Allergy Clin. Immunol. doi: 10.1016/j.jaci.2020.04.021. [Epub ahead of print].
Ling, Y., Xu, S. B., Lin, Y. X., Tian, D., Zhu, Z. Q., Dai, F. H., et al. (2020). Persistence and clearance of viral RNA in 2019 novel coronavirus disease rehabilitation patients. China Med. J. 133, 1039–1043. doi: 10.1097/CM9.000000000000774
Lippi, G., and Henry, B. M. (2020). Eosinophil count in severe coronavirus disease 2019 (COVID-19). QJM. 21:hcaa137. doi: 10.1093/qjmed/hcaa137
Lippi, G., and Plebani, M. (2020). Laboratory abnormalities in patients with COVID-2019 infection. Clin. Chem. Lab. Med. doi: 10.1515/cclm_2020-0198. [Epub ahead of print].
Liu, F., Xu, A., Zhang, Y., Xuan, W., Yan, T., Pan, K., et al. (2020). Patients of COVID-19 may benefit from sustained lopinariv-combined regimen and the increase of eosinophil may predict the outcome of COVID-19 progression. Int. J. Infect. Dis. 95, 183–191. doi: 10.1016/j.ijid.2020.03.013
Liu, K., Chen, Y., Lin, R., and Han, K. (2020). Clinical feature of COVID-19 in elderly patients: a comparison with young and middle-aged patients. J. Infect. 80, e14–e18. doi: 10.1016/j.jinf.2020.03.005
Liu, Y., Pang, Y., Hu, Z., Wu, M., Wang, C., Feng, Z., et al. (2020b). Thymosin alpha 1 (Tα1) reduces the mortality of severe COVID-19 by restoration of lymphocytopenia and reversion of exhausted T cells. Clin. Infect. Dis. ciaa630. doi: 10.1093/cid/ciaa630
Liu, Y., Yang, Y., Zhang, C., Huang, F., Wang, F., Yuan, J., et al. (2020a). L. Clinical and biochemical indexes from 2019-nCoV infected patients linked to viral load and lung injury. Sci. China Life. Sci. 63, 363–374. doi: 10.1007/s11427-020-1643-8
Lupia, T., Scabini, S., Pinna, S. M., Di Perri, G., De Rosa, F. G., and Corcione, S. (2020). 2019-novel coronavirus outbreak: a new challenge. J. Glob. Antimicr. Res. 21, 22–27. doi: 10.1016/j.gar.2020.02.021
Marik, P. E., Khangoora, V., Rivera, R., Hooper, M. H., and Catravas, J. (2017). Hydrocortisone, vitamin C, and thiamine for the treatment of severe sepsis and septic shock: a retrospective before-after study. Chest. 151, 1229–1238. doi: 10.1016/j.chest.2016.11.036
Marik, P. E., Kory, P., and Varon, J. (2020). Does vitamin D status impact mortality from SARS-CoV-2 infection? Med. Drug. Disc. 6:100041.
Mehta, P., McAuley, D. F., Brown, M., Sanchez, E., Tattersall, R. S., and Manson, J. J. (2020). COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet 395, 1033–1034. doi: 10.1016/S0140-6736(20)30628-0
Michaelis, M., Doerr, H. W., and Cinatl, J. (2011). Investigation of the influence of EPS® 7630, a herbal drug preparation from Pelargonium sidoides, on replication of a broad panel of respiratory viruses. Phytomedicine 18, 384–386. doi: 10.1016/j.phymed.2010.09.008
Mo, P., Xing, Y., Xiao, Y., Deng, L., Zhao, Q., Wang, H., et al. (2020). Clinical characteristics of refractory COVID-19 pneumonia in Wuhan, China. Clin. Infect. Dis. doi: 10.1093/cid/ciaa270. [Epub ahead of print].
Murphy, E. J., Masterson, C., Rezoagli, E., O'Toole, D., Major, I., Stack, G. D., et al. (2020). β-Glucan extract from the same edible shiitake mushroom Lentinus edodes produce differential in-vitro immunomodulatory and pulmonary cytoprotective effects – implications for coronavirus disease (COVID-19) immunotherapies. Sci. Total. Environ. 732:139330. doi: 10.1016/j.scitotenv.2020.139330
Nabzdyk, C. S., and Bittner, E. A. (2018). Vitamin C in the critically ill – indications and controversies. World J. Crit. Care Med. 7, 52–61. doi: 10.5492/wjccm.v7.i5.52
O'Brien, T. R., Thomas, D. L., Jackson, S. S., Prokunina-Olsson, L., Donnelly, R. P., and Hartmann, R. (2020). Weak induction of interferon expression by SARS-CoV-2 supports clinical trials of interferon lambda to treat early COVID-19. Clin. Infect. Dis. 17:ciaa453. doi: 10.1093/cid/ciaa453
Ouyang, Y., Yin, J., Wang, W., Shi, H., Shi, Y., Xu, B., et al. (2020). Down-regulated gene expression spectrum and immune responses changed during the disease progression in COVID-19 patients. Clin. Infect. Dis. 70:ciaa462. doi: 10.1093/cid/ciaa462
Peng, Y. D., Meng, K., Guan, H. Q., Leng, L., Zhu, R. R., Wang, B. Y., et al. (2020). Cheng LX, Huang K, Zeng QT. Clinical characteristics and outcomes of 112 cardiovascular disease patients infected by 2019-nCoV. Zhonghua. Xin. Xue. Guan. Bing. Za. Zhi. 48:E004. doi: 10.3760/cma.j.cn112148-20200220-00105
Qian, G. Q., Yang, N. B., Ding, F., Ma, A. H. Y., Wang, Z. Y., Shen, Y. F., et al. (2020). Epidemiologic and clinical characteristics of 91 hospitalized patients with COVID-19 in Zhejiang, China: a retrospective, multi-centre case series. QJM. 17:hcaa089. doi: 10.1093/qjmed/hcaa089
Qilin, L., Ding, X., Xia, G., Chen, H. G., Chen, F., Chen, Z, et al. (2020). Eosinopenia and elevated C-reactive protein facilitate triage of COVID-19 patients in fever clinic: a retrospective case-control study. EClinicalMedicine 3:100375. doi: 10.1016/j.eclinm.2020.100375
Qin, C., Zhou, L., Hu, Z., Zhang, S., Yang, S., Tao, Y., et al. (2020). Dysregulation of immune response in patients with COVID-19 in Wuhan, China. Clin. Infect. Dis. 12:ciaa248. doi: 10.1093/cid/ciaa248
Qu, R., Ling, Y., Zhang, Y. H., We, L. Y., Chen, X., Li, X., et al. (2020). Platelet-to-lymphocyte ratio is associated with prognosis in patients with Corona Virus Diseases-19. J. Med. Virol. doi: 10.1002/jmv.25767. [Epub ahead of print].
Ruan, Q., Yang, K., Wang, W., Jiang, L., and Song, J. (2020). Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intens. Care Med. 46, 846–848. doi: 10.1007/s00134-020-05991-x
Shi, Y., Wang, Y., Shao, C., Huang, J., Gan, J., Huang, X., et al. (2020). COVID-19 infection: the perspectives on immune responses. Cell Death. Differ. 27, 1451–1454. doi: 10.1038/s41418-020-0530-3
Shin, H. S., Kim, Y., Kim, G., Lee, J. Y., Jeong, I., Joh, J. S., et al. (2019). Immune responses to Middle-East respiratory syndrome coronavirus during the acute and convalescent phases of human infection. Clin. Infect. Dis. 68, 984–992. doi: 10.1093/cid/ciy595
Shitti, M. O., and Afolami, O. I. (2020). Improving the efficacy of chloroquine and hydroxychloroquine against SARS-CoV2 may require zinc additives – a better synergy for future COVID-19 clinical trials. Le Infezi. Med. 2, 192–197.
Silberstein, M. (2020). Vitamin D: a simpler alternative to tocilizumab for trial in COVID-19? Med. Hypothes. 140:109767. doi: 10.1016/j.mehy.2020.109767
Skalny, A. V., Rink, L., Ajsuvakova, O. P., Aschner, M., Gritsenko, V. A., Alekseenko, S. I., et al. (2020). Zinc and respiratory tract infections: perspectives for COVID-19 (Review). Int. J. Mol. Med. 46, 17–26. doi: 10.3892/ijmm.2020.4757
Sun, D. W., Zhang, D., Tian, R. H., Li, Y., Wang, Y. S., Cao, J., et al. (2020). The underlying changes and predicting role of peripheral blood inflammatory cells in severe COVID-19 patients: a sentinel? Clin. Chim. Acta. 508, 122–129. doi: 10.1016/j.cca.2020.05.027
Sun, S., Cai, X., Wang, H., He, G., Lin, Y., Lu, B., et al. (2020). Abnormalities of peripheral blood system in patients with COVID-19 in Wenzhou, China. Clin. Chim. Acta. 507, 174–180. doi: 10.1016/j.cca.2020.04.024
Te Velthuis, A. J. W., van den Worm, S. H. E., Sims, A. C., Baric, R. S., Snijder, E. J., and van Hemert, M. J. (2010). Zn2+ inhibits coronavirus and arterivirus RNA polymerase activity in vitro and zinc ionophores block the replication of these viruses in cell culture. PLoS Pathog. 6:e1001176. doi: 10.1371/journal.ppat.1001176
Thevarajan, I., Nguyen, T. H. O., Koutsakos, M., Druce, J., Caly, L., van de Sandt, C. E., et al. (2020). Breadth of concomitant immune responses prior to patient recovery: a case report of non-severe COVID-19. Nat. Med. 26, 453–455. doi: 10.1038/s41591-020-0819-2
Urbancikova, I., Hudackova, D., Majtan, J., Rennerova, Z., Banovcin, P., and Jesenak, M. (2020). Efficacy of Pleuran (β-glucan from Pleurotus ostreatus) in the management of herpes simplex virus type 1 infection. Evid. Bas. Complem. Altern. Med. 2020:8562309. doi: 10.1155/2020/8562309
Vardhana, S. A., and Wolchok, J. D. (2020). The many faces of the anti-COVID immune response. J. Exp. Med. 217:e20200678. doi: 10.1084/jem.20200678
Wan, S., Yiang, Y., Fang, W., Zheng, Y., Li, B., Hu, Y., et al. (2020). Clinical features and treatment of COVID-19 patients in Northeast Chongqing. J. Med. Virol. 92:797–806. doi: 10.1002/jmv.25783
Wang, F., Hou, H., Luo, Y., Tang, G., Wu, S., Huang, M., et al. (2020). The laboratory tests and host immunity of COVID-19 patients with different severity of illness. J. Clin. Immunol. Insight. 5:e137799. doi: 10.1172/jci.insight.137799
Wang, Z., Yang, B., Li, Q., Wen, L., and Zhang, R. (2020). Clinical features of 69 cases with coronavirus disease 2019 in Wuhan, China. Clin. Inf. Dis. 16:ciaa272. doi: 10.1093/cid/ciaa272
Wen, W., Su, W., Tang, H., Le, W., Zhang, X., Zheng, Y., et al. (2020). Immune cell profiling of COVID-19 patients in the recovery stage by single-cell sequencing. Cell Discov. 6:31. doi: 10.1038/s41421-020-0168-9
Wu, C., Chen, X., Cai, Y., Xia, J., Zhou, X., Xu, S., et al. (2020). Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA. Intern. Med. 13:e200994. doi: 10.1001/jamainternmed.2020.0994
Wu, D., and Yang, X. O. (2020). TH17 responses in cytokine storm of COVID-19: an emerging target of JAK2 inhibitor fedratinib. J. Microbiol. Immunol. Infect. 53, 368–370. doi: 10.1016/j.jmii.2020.03.005
Wu, Z., and McGoogan, J. M. (2020). Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China. summary of a report of 72 314 cases from the Chinese center for disease control and prevention. JAMA. doi: 10.1001/jama.2020.2648. [Epub ahead of print].
Xu, B., Fan, C. Y., Wang, A. I., Zou, Y. L., Yu, Y. H., He, C., et al. (2020). Suppressed T cell-mediated immunity in patients with COVID-19: a clinical retrospective study in Wuhan, China. J. Infect. 81, e51–e60. doi: 10.1016/j.jinf.2020.04.012
Yang, W., Cao, Q., Qin, L., Wang, X., Cheng, Z., Pan, A., et al. (2020). Clinical characteristics and imaging manifestations of the 2019 novel coronavirus disease (COVID-19): a multi-center study in Wenzhou city, Zhejiang, China. J. Infect. 86, 388–393. doi: 10.1016/j.jinf.2020.02.016
Yao, X. H., Li, T. Y., He, Z. C., Ping, Y. F., and Liu, H. W. (2020). A pathological report of three COVID-19 cases by minimally invasive autopsies. Zhonghua. Bing. Li. Xue. Za. Zhi. 49:E009. doi: 10.3760/cma.j.cn112151-20200312-00193
Ye, Q., Wang, B., and Mao, J. (2020). The pathogenesis and treatment of the ‘cytokine storm’ in COVID-19. J. Infect. 80, 607–613. doi: 10.1016/j.jinf.2020.03.037
Yi, Y., Lagniton, P. N. P., Ye, S., Li, E., and Xu, E. H. (2020). COVID-19: what has been learned and to be learned about the novel coronavirus disease. Int. J. Biol. Sci. 16:1753–1766. doi: 10.7150/ijbs.45134
Yun, H., Sun, Z., Wu, J., Tang, A., Hu, M., and Xiang, Z. (2020). Laboratory data analysis of novel coronavirus (COVID-2019) screening in 2510 patients. Clin. Chim. Acta. 507, 94–97. doi: 10.1016/j.cca.2020.04.018
Zeng, Q., Li, Y. Z., Huang, G., Wu, W., Dong, S. Y., and Xu, Y. (2020). Mortality of COVID-19 is associated with cellular immune function compared to immune function in Chinese Han population. medRxiv. doi: 10.1101/2020.03.08.20031229. [Epub ahead of print].
Zhang, D. H., Wu, K. I., Zhang, X., Deng, S. Q., and Peng, B. (2020). In silico screening of Chinese herbal medicines with the potential to directly inhibit 2019 novel coronavirus. J. Integr. Med. 18, 152–158. doi: 10.1016/j.joim.2020.02.005
Zhang, J. J., Dong, X., Cao, Y. Y., Yuan, Y. D., Yang, Y. B., Yan, Y. Q., et al. (2020). Clinical characteristics of 140 patient infected with SARS-CoV-2 in Wuhan, China. Allergy. doi: 10.1111/all.14328. [Epub ahead of print].
Zhang, M. Q., Wang, X. H., Chen, Y. L., Zhao, K. L., Cai, Y. Q., An, C. L., et al. (2020). Clinical features of 2019 novel coronavirus pneumonia in the early stage from a fever clinic in Beijing. Zhonghua. Jie. He. He. Hu. Xi. Za. Zhi. 43, 215–218. doi: 10.3760/cma.j.issn.1001-0939.2020.0013
Zhang, X., Cai, H., Hu, J., Lian, J., Gu, J., Zhang, S., et al. (2020). Epidemiological, clinical characteristics of cases of SARS-CoV-2 infection with abnormal imaging findings. Int. J. Inf. Dis. 94, 81–87. doi: 10.1016/j.ijid.2020.03.040
Zheng, M., Gao, Y., Wang, G., Song, G., Liu, S., Sun, D., et al. (2020). Functional exhaustion of antiviral lymphocytes in COVID-19 patients. Cell. Mol. Immunol. 17, 533–535. doi: 10.1038/s41423-020-0402-2
Zhou, Y., Han, T., Chen, J., Hou, C., Hua, L., He, L., et al. (2020a). Clinical and autoimmune characteristics of severe and critical cases with COVID-19. Clin. Transl. Sci. doi: 10.1111/CTS.12805. [Epub ahead of print].
Keywords: coronavirus 2019, SARS-CoV2, COVID-19, lymphopenia, eosinopenia, cytokine storm, immunoparalysis
Citation: Jesenak M, Brndiarova M, Urbancikova I, Rennerova Z, Vojtkova J, Bobcakova A, Ostro R and Banovcin P (2020) Immune Parameters and COVID-19 Infection – Associations With Clinical Severity and Disease Prognosis. Front. Cell. Infect. Microbiol. 10:364. doi: 10.3389/fcimb.2020.00364
Received: 31 March 2020; Accepted: 12 June 2020;
Published: 30 June 2020.
Edited by:
Stephanie M. Seveau, The Ohio State University, United StatesReviewed by:
Robert Cody Sharp, University of Florida Health, United StatesPenghua Wang, University of Connecticut Health Center, United States
Copyright © 2020 Jesenak, Brndiarova, Urbancikova, Rennerova, Vojtkova, Bobcakova, Ostro and Banovcin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Milos Jesenak, jesenak@gmail.com; Miroslava Brndiarova, mirkabdc@gmail.com; Zuzana Rennerova, zuzana.rennerova@yahoo.com
 Milos Jesenak
Milos Jesenak Miroslava Brndiarova1*
Miroslava Brndiarova1*  Zuzana Rennerova
Zuzana Rennerova Jarmila Vojtkova
Jarmila Vojtkova