Abstract
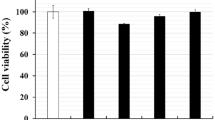
Outer inflammatory protein (OipA) is an important virulence factor of Helicobacter pylori (H. pylori), but the correlation between oipA copy number and its virulence remains unknown. The study was designed to investigate whether the duplicate oipA gene loci showed more virulent than one oipA gene in vitro. H. pylori strain CCS9803 (China Chongqing Strain 9803) that carries duplicate oipA loci was used to construct one or two oipA knockout mutant strain, which was further verified by qPCR and western blot. Gastric epithelial cells AGS and GES-1 were infected with wild-type (WT) or oipA mutants for 6 or 24 h. The expression levels of IL-8, bacterial adhesion, cell apoptosis and cell cycle were performed to analyze the function of oipA. The WT and oipA mutant strains induce significantly higher mRNA and protein levels of IL-8 than the uninfected group (P < 0.05), but only oipA2 mutants induced significantly decreased expression levels than the WT-infected group (P < 0.05). Adherence to gastric cells was significantly decreased by inactivated two oipA loci (P < 0.05). The WT strain caused a significant rising proportion of early apoptosis cell, which had dropped after duplicate oipA genes were both knockout (P < 0.05). WT and oipA1 mutants failed to affect cell cycle; however, the oipA2 mutants increased M phase and reduced S phase when compared to the uninfected group. In conclusion, our study demonstrated that oipA impacts IL-8 expression, adherence, cell apoptosis and cell cycle of gastric cells independent of its gene copy number.





Similar content being viewed by others
Data availability
All data in the study will be available if requested.
References
Marshall BJ, Warren JR (1984) Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet 1(8390):1311–1315. https://doi.org/10.1016/s0140-6736(84)91816-6
Kusters JG, van Vliet AH, Kuipers EJ (2006) Pathogenesis of Helicobacter pylori infection. Clin Microbiol Rev 19(3):449–490. https://doi.org/10.1128/CMR.00054-05
Blaser MJ (1998) Helicobacter pylori and gastric diseases. BMJ 316(7143):1507–1510. https://doi.org/10.1136/bmj.316.7143.1507
Schistosomes, liver flukes and Helicobacter pylori (1994) IARC Working Group on the evaluation of carcinogenic risks to humans. Lyon, 7–14 June 1994. IARC Monogr Eval Carcinog Risks Hum 61:1–241
Choi IJ, Kook MC, Kim YI, Cho SJ, Lee JY, Kim CG, Park B, Nam BH (2018) Helicobacter pylori therapy for the prevention of metachronous gastric cancer. N Engl J Med 378(12):1085–1095. https://doi.org/10.1056/NEJMoa1708423
Mera RM, Bravo LE, Camargo MC, Bravo JC, Delgado AG, Romero-Gallo J, Yepez MC, Realpe JL, Schneider BG, Morgan DR, Peek RM Jr, Correa P, Wilson KT, Piazuelo MB (2018) Dynamics of Helicobacter pylori infection as a determinant of progression of gastric precancerous lesions: 16-year follow-up of an eradication trial. Gut 67(7):1239–1246. https://doi.org/10.1136/gutjnl-2016-311685
Wong BC, Lam SK, Wong WM, Chen JS, Zheng TT, Feng RE, Lai KC, Hu WH, Yuen ST, Leung SY, Fong DY, Ho J, Ching CK, Chen JS (2004) Helicobacter pylori eradication to prevent gastric cancer in a high-risk region of China: a randomized controlled trial. JAMA 291(2):187–194. https://doi.org/10.1001/jama.291.2.187
Fukase K, Kato M, Kikuchi S, Inoue K, Uemura N, Okamoto S, Terao S, Amagai K, Hayashi S, Asaka M (2008) Effect of eradication of Helicobacter pylori on incidence of metachronous gastric carcinoma after endoscopic resection of early gastric cancer: an open-label, randomised controlled trial. Lancet (London, England) 372(9636):392–397. https://doi.org/10.1016/s0140-6736(08)61159-9
Ohnishi N, Yuasa H, Tanaka S, Sawa H, Miura M, Matsui A, Higashi H, Musashi M, Iwabuchi K, Suzuki M, Yamada G, Azuma T, Hatakeyama M (2008) Transgenic expression of Helicobacter pylori CagA induces gastrointestinal and hematopoietic neoplasms in mouse. Proc Natl Acad Sci USA 105(3):1003–1008. https://doi.org/10.1073/pnas.0711183105
Amieva MR, Vogelmann R, Covacci A, Tompkins LS, Nelson WJ, Falkow S (2003) Disruption of the epithelial apical-junctional complex by Helicobacter pylori CagA. Science 300(5624):1430–1434. https://doi.org/10.1126/science.1081919
Murata-Kamiya N, Kurashima Y, Teishikata Y, Yamahashi Y, Saito Y, Higashi H, Aburatani H, Akiyama T, Peek RM Jr, Azuma T, Hatakeyama M (2007) Helicobacter pylori CagA interacts with E-cadherin and deregulates the beta-catenin signal that promotes intestinal transdifferentiation in gastric epithelial cells. Oncogene 26(32):4617–4626. https://doi.org/10.1038/sj.onc.1210251
Cover TL, Krishna US, Israel DA, Peek RM Jr (2003) Induction of gastric epithelial cell apoptosis by Helicobacter pylori vacuolating cytotoxin. Can Res 63(5):951–957
Kim I-J, Lee J, Oh SJ, Yoon M-S, Jang S-S, Holland RL, Reno ML, Hamad MN, Maeda T, Chung HJ, Chen J, Blanke SR (2018) Helicobacter pylori infection modulates host cell metabolism through VacA-dependent inhibition of mTORC1. Cell Host Microbe 23(5):583–593.e588. https://doi.org/10.1016/j.chom.2018.04.006
Yamaoka Y, Ojo O, Fujimoto S, Odenbreit S, Haas R, Gutierrez O, El-Zimaity HM, Reddy R, Arnqvist A, Graham DY (2006) Helicobacter pylori outer membrane proteins and gastroduodenal disease. Gut 55(6):775–781. https://doi.org/10.1136/gut.2005.083014
Alm RA, Bina J, Andrews BM, Doig P, Hancock RE, Trust TJ (2000) Comparative genomics of Helicobacter pylori: analysis of the outer membrane protein families. Infect Immun 68(7):4155–4168. https://doi.org/10.1128/iai.68.7.4155-4168.2000
Borén T, Falk P, Roth KA, Larson G, Normark S (1993) Attachment of Helicobacter pylori to human gastric epithelium mediated by blood group antigens. Science (New York, NY) 262(5141):1892–1895. https://doi.org/10.1126/science.8018146
Mahdavi J, Sonden B, Hurtig M, Olfat FO, Forsberg L, Roche N, Angstrom J, Larsson T, Teneberg S, Karlsson KA, Altraja S, Wadstrom T, Kersulyte D, Berg DE, Dubois A, Petersson C, Magnusson KE, Norberg T, Lindh F, Lundskog BB, Arnqvist A, Hammarstrom L, Boren T (2002) Helicobacter pylori SabA adhesin in persistent infection and chronic inflammation. Science 297(5581):573–578. https://doi.org/10.1126/science.1069076
Kennemann L, Brenneke B, Andres S, Engstrand L, Meyer TF, Aebischer T, Josenhans C, Suerbaum S (2012) In vivo sequence variation in HopZ, a phase-variable outer membrane protein of Helicobacter pylori. Infect Immun 80(12):4364–4373. https://doi.org/10.1128/IAI.00977-12
Zhao Q, Song C, Wang K, Li D, Yang Y, Liu D, Wang L, Zhou N, Xie Y (2020) Prevalence of Helicobacter pylori babA, oipA, sabA, and homB genes in isolates from Chinese patients with different gastroduodenal diseases. Med Microbiol Immunol. https://doi.org/10.1007/s00430-020-00666-2
Yamaoka Y, Kwon DH, Graham DY (2000) A M(r) 34,000 proinflammatory outer membrane protein (oipA) of Helicobacter pylori. Proc Natl Acad Sci USA 97(13):7533–7538. https://doi.org/10.1073/pnas.130079797
Farzi N, Yadegar A, Aghdaei HA, Yamaoka Y, Zali MR (2018) Genetic diversity and functional analysis of oipA gene in association with other virulence factors among Helicobacter pylori isolates from Iranian patients with different gastric diseases. Infect Genet Evol 60:26–34. https://doi.org/10.1016/j.meegid.2018.02.017
Snyder LAS, Loman NJ, Linton JD, Langdon RR, Weinstock GM, Wren BW, Pallen MJ (2010) Simple sequence repeats in Helicobacter canadensis and their role in phase variable expression and C-terminal sequence switching. BMC Genomics. https://doi.org/10.1186/1471-2164-11-67
Yamaoka Y, Kikuchi S, el-Zimaity HMT, Gutierrez O, Osato MS, Graham DY (2002) Importance of Helicobacter pylori oipA in clinical presentation, gastric inflammation, and mucosal interleukin 8 production. Gastroenterology 123(2):414–424. https://doi.org/10.1053/gast.2002.34781
Kudo T, Nurgalieva ZZ, Conner ME, Crawford S, Odenbreit S, Haas R, Graham DY, Yamaoka Y (2004) Correlation between Helicobacter pylori OipA protein expression and oipA gene switch status. J Clin Microbiol 42(5):2279–2281. https://doi.org/10.1128/jcm.42.5.2279-2281.2004
Al-Maleki AR, Loke MF, Lui SY, Ramli NSK, Khosravi Y, Ng CG, Venkatraman G, Goh KL, Ho B, Vadivelu J (2017) Helicobacter pylori outer inflammatory protein A (OipA) suppresses apoptosis of AGS gastric cells in vitro. Cell Microbiol. https://doi.org/10.1111/cmi.12771
Franco AT, Johnston E, Krishna U, Yamaoka Y, Israel DA, Nagy TA, Wroblewski LE, Piazuelo MB, Correa P, Peek RM Jr (2008) Regulation of gastric carcinogenesis by Helicobacter pylori virulence factors. Cancer Res 68(2):379–387. https://doi.org/10.1158/0008-5472.CAN-07-0824
Kraft C, Suerbaum S (2005) Mutation and recombination in Helicobacter pylori: mechanisms and role in generating strain diversity. Int J Med Microbiol 295(5):299–305. https://doi.org/10.1016/j.ijmm.2005.06.002
Falush D, Kraft C, Taylor NS, Correa P, Fox JG, Achtman M, Suerbaum S (2001) Recombination and mutation during long-term gastric colonization by Helicobacter pylori: estimates of clock rates, recombination size, and minimal age. Proc Natl Acad Sci USA 98(26):15056–15061. https://doi.org/10.1073/pnas.251396098
Suerbaum S, Josenhans C (2007) Helicobacter pylori evolution and phenotypic diversification in a changing host. Nat Rev Microbiol 5(6):441–452. https://doi.org/10.1038/nrmicro1658
Kawai M, Furuta Y, Yahara K, Tsuru T, Oshima K, Handa N, Takahashi N, Yoshida M, Azuma T, Hattori M, Uchiyama I, Kobayashi I (2011) Evolution in an oncogenic bacterial species with extreme genome plasticity: Helicobacter pylori East Asian genomes. BMC Microbiol 11:104. https://doi.org/10.1186/1471-2180-11-104
Furuta Y, Kawai M, Yahara K, Takahashi N, Handa N, Tsuru T, Oshima K, Yoshida M, Azuma T, Hattori M, Uchiyama I, Kobayashi I (2011) Birth and death of genes linked to chromosomal inversion. Proc Natl Acad Sci USA 108(4):1501–1506. https://doi.org/10.1073/pnas.1012579108
Draper JL, Hansen LM, Bernick DL, Abedrabbo S, Underwood JG, Kong N, Huang BC, Weis AM, Weimer BC, van Vliet AH, Pourmand N, Solnick JV, Karplus K, Ottemann KM (2017) Fallacy of the unique genome: sequence diversity within single Helicobacter pylori strains. mBio. https://doi.org/10.1128/mBio.02321-16
Jang S, Su H, Blum FC, Bae S, Choi YH, Kim A, Hong YA, Kim J, Kim JH, Gunawardhana N, Jeon YE, Yoo YJ, Merrell DS, Ge L, Cha JH (2017) Dynamic expansion and contraction of cagA copy number in Helicobacter pylori impact development of gastric disease. mBio. https://doi.org/10.1128/mBio.01779-16
Hong Y, Mao XH, Zeng WK, Ma LM, Jing SR, Zou QM (2005) Restriction fragment length polymorphism of adhesin gene hpaA from different Helicobacter pylori strains of Chongqing, China. World J Gastroenterol 11(17):2647–2652. https://doi.org/10.3748/wjg.v11.i17.2647
Shi Y, Liu XF, Zhuang Y, Zhang JY, Liu T, Yin Z, Wu C, Mao XH, Jia KR, Wang FJ, Guo H, Flavell RA, Zhao Z, Liu KY, Xiao B, Guo Y, Zhang WJ, Zhou WY, Guo G, Zou QM (2010) Helicobacter pylori-induced Th17 responses modulate Th1 cell responses, benefit bacterial growth, and contribute to pathology in mice. J Immunol 184(9):5121–5129. https://doi.org/10.4049/jimmunol.0901115
Fazeli Z, Alebouyeh M, Rezaei Tavirani M, Azimirad M, Yadegar A (2016) Helicobacter pylori CagA induced interleukin-8 secretion in gastric epithelial cells. Gastroenterol Hepatol Bed Bench 9(Suppl1):S42–S46
Cha B, Lim JW, Kim H (2015) Jak1/Stat3 is an upstream signaling of NF-kappaB activation in Helicobacter pylori-induced IL-8 production in gastric epithelial AGS cells. Yonsei Med J 56(3):862–866. https://doi.org/10.3349/ymj.2015.56.3.862
Talarico S, Whitefield SE, Fero J, Haas R, Salama NR (2012) Regulation of Helicobacter pylori adherence by gene conversion. Mol Microbiol 84(6):1050–1061. https://doi.org/10.1111/j.1365-2958.2012.08073.x
Teymournejad O, Mobarez AM, Hassan ZM, Talebi Bezmin Abadi A (2017) Binding of the Helicobacter pylori OipA causes apoptosis of host cells via modulation of Bax/Bcl-2 levels. Sci Rep 7(1):8036. https://doi.org/10.1038/s41598-017-08176-7
Acknowledgements
We thank Professor QM Zou (the third military medical university, China) for kindly providing H. pylori CCS9803 WT strain for our experiment. We thank Professor HK BI (Department of pathogenic biology, Nanjing Medical University, China) for kindly gifting us H. pylori G27 for our experiment. We thank Shanghai YH biotechnology co., LTD for cooperation with the oipA gene knockout work. We thank ABclonal Biotechnology co., Ltd. for preparation of anti-recombinant OipA antisera E12446.
Funding
The work was supported by the National Key Research and Development Program of China (No. 2016YFC1302201), National Natural Science Foundation of China (No. 81970502, No. 81860107, No. 81260076) and Leading Talent Training Plan of the Gan-Po Outstanding Talents 555 Project of Jiangxi Province (2010-3-61).
Author information
Authors and Affiliations
Contributions
XY and ZQY designed the research; ZQY wrote the manuscripts; ZQY and ZRL carried out verification experiments of knockout oipA gene and bacterial and cell culture; YWZ and WFF contributed to HCS experiments of cell apoptosis and cell cycle. WH and RJF contributed to bacterial protein extraction and western blot analysis. WYH and SCH contributed to data statistics and paper review. All the authors read and agreed to the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they do not have any competing interests.
Ethics approval
Not applicable.
Consent to participate and publication
Not applicable.
Code availability
Not applicable.
Additional information
Edited by Volkhard A.J. Kempf.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhao, Q., Yin, W., Zhao, R. et al. Outer inflammatory protein of Helicobacter pylori impacts IL-8 expression, adherence, cell apoptosis and cell cycle of gastric cells independent of its copy number. Med Microbiol Immunol 209, 621–630 (2020). https://doi.org/10.1007/s00430-020-00688-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00430-020-00688-w