Abstract
Interspecific communication between dogs and humans enables dogs to occupy significant roles in human society, both in companion and working roles. Dogs excel at using human communicative signals in problem-solving tasks, and solicit human contact when unable to solve a problem. Dogs’ sociocognitive behavior likely results from a selection for attention to humans during domestication, but is highly susceptible to environmental factors. Training for particular tasks appears to enhance dog–human communication, but effects may depend on the nature of the relationship determined by their role. Our aim was to examine two types of social cognition (responsiveness to human gestures, and human-directed communicative behavior in an unsolvable task) in pet dogs (n = 29) and detection dogs (n = 35). The groups did not differ in their ability to follow human signals, but pets were less responsive to signals given by a stranger than by their owner. Pets also exhibited more human-directed gazing in the unsolvable task, showing a bias for gazing at their owner compared with the stranger, whereas detection dogs showed greater persistence in attempting to solve the task compared with pets. Thus, different aspects of dogs’ sociocognitive behavior may differentially vary as a function of selection or training for particular roles.
Similar content being viewed by others
Domestic dogs (Canis familiaris) occupy significant roles in human society, both as companion animals integrated into the social network of the human family as well as in various occupational roles benefiting human welfare (Helton, 2009; Miklósi, 2015). Dogs’ remarkable adaptation to anthropocentric environments is reflected in their enhanced sensitivity to human social signals. For example, dogs are able to respond to a wide range of human communicative gestures (Kaminski & Nitzschner, 2013), discriminate human attentional states (Call, Bräuer, Kaminski, & Tomasello, 2003), and facial expressions (Müller, Schmitt, Barber, & Huber, 2015), and are capable of social learning from human demonstrators (Huber, Popovová, Riener, Salobir, & Cimarelli, 2018; Pongrácz et al., 2001).
The relative contributions of ontogeny and phylogeny in shaping dogs’ social cognition have been an active focus of canine social cognition research, giving rise to a plethora of studies examining differences in sociocognitive abilities within and between different canid groups. The object-choice task (OCT) is widely used to measure differences in dogs’ responsiveness to human communicative gestures (namely, pointing) as a function of genetic and life history. In this task, a human signals the correct location of a hidden reward (e.g., by pointing to it). Considerable research has shown that dogs are capable of following a human pointing gesture to locate hidden rewards (Kaminski & Nitzschner, 2013; Reid, 2009), though choices appear to be guided by the general direction indicated by a protruding body part rather than the direction indicated by the pointing index finger (Lakatos, Soproni, Doka, & Miklósi, 2009), as well as more subtle cues like head turning (McKinley & Sambrook, 2000) and gaze direction (Soproni, Miklósi, Topál, & Csányi, 2001). While the OCT measures dogs’ ability to comprehend communicative signals delivered by a human, the unsolvable task (UT), sometimes referred to as the impossible task, measures dogs’ tendency to emit communicative signals toward a human. In the UT, dogs are presented with an inaccessible reward, such as a treat, trapped inside a locked container. Communicative signals emitted by the dog toward a nearby human, such as gazing, are considered a request for assistance. Studies finding increased sociocognitive behaviors in dogs compared with wolves (Hare, Brown, Williamson, & Tomasello, 2002; Werhahn, Virányi, Barrera, Sommese, & Range, 2016), or even between dogs bred for different working roles (Gácsi, McGreevy, Kara, & Miklósi, 2009; Udell, Ewald, Dorey, & Wynne, 2014; Wobber, Hare, Koler-Matznick, Wrangham, & Tomasello, 2009), suggest that such behaviors are the result of a selection for enhanced human-directed social sensitivity that occurred during domestication or artificial selection. However, studies demonstrating effects of other factors on both dogs’ and wolves’ performance, such as experimental conditions (Udell, Dorey, & Wynne, 2008a) and life history (D’Aniello & Scandurra, 2016; Heberlein et al., 2016; Lazarowski & Dorman, 2015; Udell, Dorey, and Wynne, 2010a, b), underscore the mediating effects of experience on the development of sociocognitive abilities.
Studies exploring the effects of prior training on sociocognitive problem solving have yielded mixed results. For example, McKinley and Sambrook (2000) found that trained gundogs were more successful in following human pointing cues in the OCT than both untrained gundogs and non-gundogs. Further, the trained dogs’ use of a more difficult cue, head orientation, improved over trials, whereas that of the untrained dogs did not, which the authors attributed to potential effects of participating in regular training on the ability to learn new tasks (i.e., “learning to learn”; Marshall-Pescini, Valsecchi, Petak, Accorsi, & Previde, 2008; McKinley & Sambrook, 2000). By contrast, Cunningham and Ramos (2014) compared groups of dogs with varying levels of training and found that pets without advanced formal training performed just as well as highly trained dogs in their ability to follow human pointing in the OCT.
Effects of training have been explored more extensively in relation to human-directed gazing behavior during problem-solving tasks. For example, studies have demonstrated increased gazing toward a human during the UT in dogs trained for activities requiring working in concert or close contact with people, such as water rescue, agility, search and rescue (SAR), and animal-assisted activity (Cavalli, Carballo, Dzik, & Bentosela, 2019; D’Aniello, Scandurra, Prato-Previde, & Valsecchi, 2015; Marshall-Pescini, Passalacqua, Barnard, Valsecchi, & Prato-Previde, 2009). Further, the degree of interaction required between handler and dog has been shown to differentially affect dogs’ gaze alternation during the UT (Marshall-Pescini et al., 2009). Dogs with agility training, which requires maintaining visual contact with handlers as they are directed through obstacles, gazed more toward a person during the UT than SAR dogs, which are trained to work at a distance from handlers. The SAR group exhibited more barking while looking back at handlers than did untrained pets and agility dogs, likely an artifact of their specific training in which they must vocally alert the handler upon a find. Conversely, guide dogs that had long been living with their handler showed greater levels of human-directed gazing during the UT than those recently completing training and still residing at the facility’s kennel, who spent a greater proportion of time attempting to independently solve the task (Scandurra, Prato-Previde, Valsecchi, Aria, & D’Aniello, 2015). Differences in dog–human communicative behaviors between dogs with different levels of training do not appear to be due to the degree of the attachment bond, leading researchers to conclude that the training that working dogs undergo does not enhance the human–dog attachment bond any more than that of typical owner–pet interactions (Mariti et al., 2013; Scandurra, Alterisio, & D’Aniello, 2016). Rather, effects of training on sociocognitive behavior in dogs likely depends on the specific nature of the task, with differential effects between training involving cooperation with people and training promoting working independently (D’Aniello & Scandurra, 2016; Scandurra et al., 2015).
Taken together, prior results suggest that different types of training and experience may have dissimilar effects on different aspects of social cognition. Substance-detection dogs, such as those trained to locate narcotics and explosives, represent a unique class of working dogs that must work effectively as part of a team with a human handler while also exhibiting a high degree of autonomy. Dependence on the handler is considered an undesirable characteristic, as it interferes with independent decision-making (Rooney, Bradshaw, & Almey, 2004). Further, the behavior and emotional state of the handler can negatively affect detection dogs’ performance (Lit, Schweitzer, & Oberbauer, 2011; Zubedat et al., 2014). On the other hand, engagement with the handler and high levels of cooperation are considered important characteristics for the successful training and operation of a detection dog (Jamieson, Baxter, & Murray, 2017). Thus, detection dogs may exhibit differing degrees of social sensitivity and independence depending on the context, and may differ compared with other types of working dogs. For example, the behavioral characteristics of a SAR dog are in many ways similar to a detection dog, with the exception that SAR dogs typically work off leash, following commands from their handler at considerable distances (Marshall-Pescini et al., 2009). Thus, SAR dogs must maintain visual contact with their handler and be highly responsive to their direction. By contrast, explosives-detection dogs typically work on leash, for the most part leading their handler, with very little guidance and ignoring social or visual cues (Lazarowski, Rogers, Waggoner, & Katz, 2019a). The demand for dogs with the necessary behavioral characteristics for performing explosives detection continues to outgrow the current supply, further exacerbated by a lack of reliable predictive measures of success (Lazarowski et al., 2018). Recent research has revealed that cognitive processes, including aspects of social cognition, are involved in detection dog performance and may serve as valuable indicators (MacLean & Hare, 2018). Therefore, a better understanding of the cognitive profile of detection dogs and how they compare with other populations also has valuable implications for improving selection and training.
We compared performance in a human-guided object-choice task and the unsolvable task between pet and detection dogs. Past research has shown mixed effects of familiarity on dogs’ behavior in the OCT and UT, with some studies showing preferential looking toward a familiar person compared with a stranger in the UT by working dogs (D’Aniello et al., 2015) and better OCT performance when the cuer was familiar in highly trained and untrained pets (Cunningham & Ramos, 2014), whereas other studies have shown no familiarity bias in the UT (D’Aniello and Scandurra, 2016) or the OCT (Scandurra et al., 2017). Therefore, we examined whether the familiarity of the person participating in the tasks influenced performance by testing dogs with a familiar (owner or handler) and unfamiliar person. We also tested dogs on two different cues varying in difficulty, ostensive cue (pointing) and a more subtle cue (head-turn), to determine whether training and experience or cuer familiarity influenced dogs’ ability to follow more difficult cues. We hypothesized that due to the high levels of trainability and tractability required in detection dogs, detection dogs would perform as well or better than pets on the OCT. On the other hand, due to a need for independent problem solving, we predicted detection dogs would exhibit lower levels of human-directed behavior during the UT.
Method
Subjects
Subjects were a group of 35 detection dogs (21 females, 14 males; mean age = 2.57 years, SEM = .29) and a group of 29 pet dogs (16 females, 13 males, mean age = 3.76 years, SEM = .58). Detection dogs were from ik9, LLC (n =31) and Auburn University’s Canine Performance Sciences (n = 4) detection dog training programs and resided in individual indoor/outdoor kennels at their respective training center. Pet dogs were recruited from Auburn University staff and students. The criteria for participation of pet dogs was that they had been living in the home of the current owner for a minimum of 1 year. Breeds in the detection-dog group included Labrador Retrievers (n = 28), Belgian Malinois (n = 1), German Shepherds (n = 2), Springer Spaniel (n = 1), German Shorthaired Pointer (n = 1), German Wirehaired Pointer–Labrador Retriever cross (n = 1), and one large-sized (approximately 60 lbs) unknown mixed breed. Breeds in the pet group included mixed breeds of unknown origins ranging from small (approximately 30 lbs) to large (approximately 70 lbs) sizes (n = 14), Labrador Retrievers (n = 4), German Shepherds (n = 2), Siberian Huskies (n = 2), Border Collies (n = 2), and one each of Australian Cattle Dog, Catahoula Leopard Dog, Great Dane, Springer Spaniel, and Standard Poodle. We categorized dogs as cooperative working breeds, defined as those originally bred for working in continuous visual contact with a human partner (e.g., herding dogs and gundogs; Gácsi et al., 2009), or noncooperative working breeds (all others) for analyses of breed effects. The Auburn University Institutional Animal Care and Use Committee approved all activities for this study.
General procedure
Testing occurred in an empty room at the Auburn University MRI Center, with the exception of two detection dogs that were tested in a room of similar size at their training facility because of transport limitations. A 2-cm dog treat (Purina® Moist & Meaty treats) was used as the reward throughout testing, except for two dogs in the pet-dog group whose owner supplied treats because of food allergies, and two dogs in the detection-dog group for which toys were used instead of food because of an indifference toward the food treats. Order of OCT and UT testing was counterbalanced across dogs (described below). For all trials, an experimenter setup and timed/scored trials and an assistant handled the dog. The dogs’ owner (pets) or trainer (detection dogs) served as the familiar person in both tasks, and a female unfamiliar experimenter served as the unfamiliar person. Though the majority of owners and handlers were female, the gender of the familiar and unfamiliar person was not always matched. Three detection dogs and six pet dogs were tested with gender-unmatched familiar (male)/unfamiliar (female) person pairs.
Object-choice task
General procedure
The OCT procedure consisted of a two-way choice task in which dogs observed a person gesture toward one of two containers to indicate the location of a hidden reward (D’Aniello et al., 2017; Hare, Call, & Tomasello, 1998; Lazarowski & Dorman, 2015; Udell, Dorey, and Wynne, 2010a, b). On each trial, the person stood approximately 2.5 m in front of the dog in the middle of two identical opaque boxes with lids (approximately 25 × 25 × 15 cm) positioned approximately 1 m apart (Fig. 1, top panel). On all trials, both boxes contained a reward to prevent dogs from relying on odor cues to locate the reward. To minimize fatigue or satiation, OCT trials were divided across two sessions occurring on 2 different days within the same week, with each session further divided into two 12-trial blocks separated by a 5-min break. Each 12-trial block consisted of 10 cued trials and tw0 control trials (i.e., no cue). The dogs’ owner (pets) or trainer (detection dogs) acted as the cuer (person performing gesture) in one of the two sessions, and an unfamiliar experimenter acted as the cuer in the other session, with the order of session/cuer familiarity counterbalanced across subjects within each group. Within each session, two different cues were performed: 10 trials of momentary distal point (pointing with index finger and full extension of the arm closest to the signaled box accompanied with a head-turn toward the box) and 10 trials of head-turn only. Five trials of each cue were presented in each 12-trial block, in pseudorandom order so that the same cue was not presented on more than two trials in a row. Blocks were counterbalanced such that the left and right sides were correct an equal number of times, with no more than two trials in a row on the same side, and each cue was presented on the left and right sides an equal number of times.
The two control trials were conducted at the end of each block to determine whether dogs were responding based on unintentional cues from any of the individuals in the room (D’Aniello et al., 2017; Jarvis & Hall, 2020; Lazarowski & Dorman, 2015). Control trials were performed in the same way as test trials, except no cue was given. One of the two boxes was predetermined as “correct”; however, choosing either box was rewarded. Above-chance performance on control trials would indicate that dogs were using extraneous cues.
Pretraining
To familiarize dogs with the test procedure and to check for motivation to participate, each block began with two pretraining trials. An assistant walked the dog on leash into the testing room and positioned the dog at the start position, gently holding the dog by the collar. The cuer showed the dog the reward and placed it in one of the two boxes, after which the assistant released the dog. If the dog approached the container the cuer had baited, the cuer opened the box and offered the reward to the dog. If the dog chose the other container, the cuer led the dog to the correct container and offered the reward. This method was repeated on the next trial for the other location (order randomized across dogs).
Testing
Immediately following pretraining, test trials began. On each trial, the cuer called the dog’s name while looking at the dog until the dog oriented forward, signaled to one of the containers using the corresponding cue for 2 s, returned to a neutral position (arms at side, facing forward, gazing straight ahead) and said “OK” to signal to the handler to release the dog, allowing 15 s to make a choice. When the dog chose a box, defined as touching or placing its nose or front paws within 15 cm of the box that the cuer signaled to, the experimenter said “choice” and recorded the response. If correct, the cuer opened the container for the dog to access the reward. If incorrect, the box was not opened, and dog was called back to the start position. If the dog did not make a choice within 15 s, the trial was terminated without repetition, and a “no choice” was recorded. No-choice trials were considered incorrect when calculating accuracy, but were also analyzed separately. If a no choice occurred on three consecutive trials, two pretraining trials (one on each side) were conducted in order to verify dogs’ motivation to continue participating and to ensure that any no-choice trials were due to a failure to follow the gesture rather than a loss of motivation to participate due to satiation or fatigue. Between trials, the assistant led the dog out of the testing room while the next trial was set up. All trials were live-scored by the experimenter and double-scored from video by an independent observer. Interrater reliability (IRR) was near perfect for the OCT (Cohen’s kappa; k = .942). Discrepancies were resolved according to the video.
Unsolvable task
The UT testing was performed on one of the two sessions following OCT trials, with the session (first or second) containing UT counterbalanced within each group. The apparatus consisted of a Sterilite® storage container (17 × 13 × 15 cm) lid mounted upside down onto a plywood base (66 × 50 × 4 cm). The container could then be placed upside down on the lid and either left unlocked so that it could be easily knocked off from its base, or locked so that it remained attached to its base. On all trials, an unfamiliar and familiar person (same individuals as OCT) stood on either side of the apparatus, approximately 1 m apart, facing each other (Fig. 1, top panel).
Prior to formal UT testing, a series of demonstration trials were performed in which dogs were shown that the container could be knocked over to reveal a reward, and the dog was encouraged to knock the apparatus. Once the dog reliably manipulated the apparatus to obtain the reward without assistance, four “solvable trials” were conducted in which the container was placed over its base, but left unlocked. On each solvable trial, the familiar and unfamiliar persons were in position before the dog entered the testing room. The assistant positioned the dog at the starting point (1 m away from apparatus) and then released the dog for 15 s or until the dog obtained the reward, whichever came first. A 30-s intertrial interval separated each trial, during which the dog was removed from the test area and the next trial was set up. Immediately following the solvable trials, four unsolvable trials were conducted in which the container was locked to its base. Unsolvable trials occurred in the same way as solvable trials, with each trial lasting 15 s.
Behaviors of interest during the unsolvable trials were duration of human-directed gazing (looking at either the unfamiliar or familiar person’s face), human-directed interaction (physical contact with either person), and persistence (physical contact with the apparatus), calculated as total percentage of time, all scored from video. To assess IRR, a second independent observer scored 60% of UT sessions. IRR between the two scorers was good (intraclass correlation coefficient [ICC]: gaze duration ICC = .762, 95% CI [.641, .842]; physical interaction ICC = .731, 95% CI [.577, .826]). Disagreement was resolved by a third observer.
Data analysis
Choices on each trial in the OCT were coded as a binary variable (correct = 1; incorrect = 0) and were analyzed by a generalized mixed-effects model (GLMM) with binomial family distribution (lme4 package; Bates, Mächler, Bolker, & Walker, 2015). Dog identity was included as a random factor to control for repeated measures. Group (detection or pet), breed group (cooperative working breed or not), cue (point, head turn, or control), and cuer familiarity (familiar or unfamiliar) were included as fixed factors as well as their interactions. Session block (four levels) and order of familiar/unfamiliar condition were included as fixed factors to assess learning over the course of the experiment and order effects, as well as their interactions with group. Sex and age were included as additional fixed factors. Significant interactions were followed up with separate GLMMs. Average accuracy for each group for each cue and condition was also compared with chance (50%) using one-sample t tests, and binomial tests were used to determine whether individual performance was significantly above chance (50%; >8/10 correct; p < 0.05). Similar, separate analyses were run to assess effects of the same factors on percentage of no-choice trials.
GLMMs with individual dog as a random factor were used to analyze effects of group, breed group, sex, age, familiarity of person, and interactions between group and familiarity on duration of each of the human-directed behaviors (i.e., gazing and physical contact) in the UT. Persistence (percentage of trial time interacting with the apparatus) was analyzed using a generalized linear model (GLM) with group, sex, and age as fixed factors.
Spearman correlations were run to examine correlations between dependent measures of the two tasks. Analyses were performed in the R statistical program (Version 1.2.5033, RStudio) and SPSS Version 25.
Results
Object-choice task
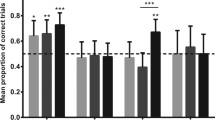
Ten detection dogs and seven pet dogs did not return for the second OCT session, resulting in a total of 25 detection dogs and 22 pet dogs in the final OCT sample. Figure 2 shows pet and detection dogs’ accuracy in each condition of the OCT. Accuracy was significantly higher on pointing (M = 68.42, SEM = 2.33) compared with head turn (M = 51.36, SEM = 1.79, GLMM: z = 7.68, p < .001) and control (i.e., no cue) trials (M = 47.6, SEM = 2.48; GLMM: z = 7.305, p < .001). There was no difference in accuracy between head-turn and control trials (z = 1.443, p = .15). Both groups were significantly above chance in following the pointing cue in the familiar, one-sample t test = detection dogs: t(24) = 4.043, p < .001; pets: t(21) = 4.805, p < .001, and unfamiliar conditions, detection dogs: t(24) = 4.522, p < .001; pets: t(21) = 2.721, p = .013. Performance was at chance levels for both groups on head-turn trials, regardless of familiarity condition (ps > .185); however, 3/22 pets and 3/25 detection dogs were significantly above chance on head-turn trials at the individual level (at least 8/10 trials correct, binomial test: p < .05).
Mean (+ SEM) percentage correct in the object-choice task as a function of cue type (pointing + head turn or head turn only) and familiarity (familiar or unfamiliar person), for pets (left panel) and detection dogs (right panel). Dashed line indicates chance (50%), and asterisks represent performance significantly above chance (p < .05). Error bars represent standard error of the mean
Breed group significantly affected overall OCT accuracy, where accuracy was higher in cooperative working breeds (M = 63.21, SEM = 9.99) than in noncooperative working breeds (M = 50.21, SEM = 2.61, GLMM: z = 3.40, p < .001). Sex, age, group, familiarity of the person giving the cue, order of condition, and session block did not significantly affect OCT accuracy (ps > .06).
Incorrect trials were further coded as having chosen the incorrect box or failing to make a choice within the 15 s allotted (i.e., a “no choice”). There was a significant effect of breed group on total percentage of no-choice trials, where cooperative working breeds committed fewer no-choices (M = 6.73, SEM = .97) than noncooperative breeds (M = 22.57, SEM = 2.98), GLMM, t(42) = −3.83, p < .001. There was also an interaction between group and condition on percentage of no-choice trials, t(231) = 2.20, p = .028, where pets committed significantly more no-choices in the unfamiliar condition (M = 17.27, SEM = 3.32) than the familiar condition (M = 6.59, SEM = 1.56), t(107) = 3.06, p = .002, with no such effect for detection dogs, t(122) = .62, p = .33 (see Fig. 3). Sex, age, group, condition, and cue did not significantly affect percentage of no-choice trials (ps > .129).
Mean (+ SEM) percentage of trials in which “no choice” was scored (dog failed to make a response within 15 s) in the familiar and unfamiliar conditions of the object-choice task for pet dogs and detection dogs. Asterisk (*) denotes a significant difference at the p < .05 level and error bars represent standard error of the mean
Controls
Performance was not significantly different from chance on control trials for detection dogs, one-sample t test: t(49)) = 1, p = .322, and was below chance for pet dogs, one-sample t test: t(43) = −2.189, p = .034, indicating that dogs were not choosing the correct container based on unintentional cues. The below-chance performance of pets was likely due to the higher incidence of no choices on control trials (20%; detection dogs: 10%), compared with 11% when a cue was given (7% for detection dogs). No choices on control trials, when no cue is given, further indicate that pets were choosing based on the cue itself, without which they did not have the information to make a choice.
Unsolvable task
Thirty detection dogs and 26 pet dogs participated in the UT; three pet dogs failed to solve the task during the demonstration trials and thus did not advance to testing, resulting in complete data for 23 pet dogs.
Figure 4 shows pet and detection dogs’ gaze duration (percentage of total trial time) toward the familiar and unfamiliar person. Pet dogs spent significantly greater percentages of the unsolvable trial engaged in human-oriented gazing (M = 4.64%, SEM = 1.11) than did detection dogs (M = 3.13%, SEM = .65), GLMM, t(48) = 2.32, p = .03. There was no overall effect of familiarity of the person (p = .80), but there was a significant interaction between group and familiarity, t(51) = −2.21, p = .032, where pets gazed longer at the familiar person than at the unfamiliar person, t(22) = −2.57, p = .02, with no such effect for detection dogs, t(29) = −.31, p = .760 (see Fig. 4). Age was also a significant factor in human-oriented gazing, such that for every 1-year increase in age, dogs gazed for .90 seconds longer, t(48) = 3.04, p = .003. Sex and breed group did not significantly affect duration of human-oriented gazing (ps > .06). There were no effects of group, sex, age, breed group, or familiarity of the person on duration of human-oriented physical interaction (ps > .11). Persistence, measured as the duration of interaction with the box, was significantly higher in detection dogs (M = 50.13%, SEM = 4.22) than in pet dogs (M = 33.70%, SEM = 4.37) GLM, t(49) = −2.293, p = .03, with no effect of sex or age (ps > .48).
Between-task correlations
Forty-five dogs completed both the OCT and UT. To determine whether there were common sociocognitive mechanisms between the tasks, overall accuracy on the OCT was correlated to overall duration of human-oriented gazing as well as human-oriented physical contact; none of these correlations were significant (ps > .1).
Discussion
The primary aim of this study was to compare the performance of pet and detection dogs on two measures of social cognition, evaluating either the comprehension of communicative signals given by a human (OCT) or the emission of human-directed communicative signals (UT). We further examined differences in sociocognitive behavior as a function of the familiarity of the human participating in the task. We predicted that pet and detection dogs would differ in their OCT and UT performance due to differences in training history and the nature of interactions with people. The results suggest that experience may differentially affect different aspects of social cognition in dogs.
There was no difference between pet and detection dogs in their ability to follow either cue in the OCT, and both groups performed significantly above chance in following pointing regardless of whether the person pointing was familiar or unfamiliar. No effects of session block were found, indicating that performance was not the result of learning over the course of the test trials. Consistent with past studies, performance was higher on pointing than on head-turning trials, which was on average not better than chance for either group (Cunningham & Ramos, 2014; Udell, Giglio, & Wynne, 2008b). Previous studies in both pet and working dogs have also found that dogs accurately follow pointing gestures independent of cuer familiarity (Miklósi, Polgárdi, Topál, & Csányi, 1998; Scandurra et al., 2017). Furthermore, detection dogs accurately followed pointing gestures at high rates despite their training in following nonvisual cues (i.e., olfaction). The lack of familiarity and group effects may be due to the pointing gesture being a rather salient and easy cue for dogs that have a significant amount of interaction with people, such that the task is not sensitive to these factors. By contrast, the head-turn cue may have been too difficult resulting in a floor effect masking potential differences. For example, on average pets performed nearly 10% better in following the head turn cue given by their owner than a stranger, though this difference was not significance due to variability in performance as a result of the majority of dogs performing at chance. Thus, the familiarity of the cuer may influence performance depending on the difficulty of the cue.
Breed group significantly influenced OCT performance, where dogs from cooperative working breeds (e.g., retrievers, herding dogs) performed more accurately overall than did dogs from other breed groups. Consistent with other studies, this finding supports the hypothesis that selection for working while maintaining visual contact with humans enhanced sensitivity and responsiveness to human gestures (Gácsi et al., 2009). However, given breed-based stereotypes and expectations, the possibility that owners of particular breeds engage in breed-specific activities with their dog makes disentangling effects of genetics and experience when evaluating breed effects challenging (Udell et al., 2014).
Despite no group differences in cue-following accuracy, pet dogs committed significantly more no-choices when the cuer was unfamiliar compared with familiar, with no such effect observed for detection dogs. One explanation could be that pet dogs were less motivated to participate, as pet dogs are less accustomed to long training sessions or to situations in which they are required to problem-solve on their own (Miklósi, 2015). However, motivation trials were included after three consecutive no-choice trials in order to ensure that dogs were willing to continue participating, and so a lack of motivation is unlikely to account for the increase in no-choice trials, particularly because they occurred more often on unfamiliar person trials. Decreased responding when the cuer was unfamiliar may be explained by findings that dogs tend to pay more attention to their owners than to unfamiliar people (Mongillo, Bono, Regolin, & Marinelli, 2010), suggesting that decreased responding on unfamiliar trials by pets were due to a lack of attention to the information required to solve the task. The fact that this was only seen in pets suggests that detection dogs flexibly respond to people regardless of familiarity, which is important for dogs that are likely to work with various trainers and handlers throughout their career. Similarly, breeds selected for working closely with humans are reportedly more attentive toward humans than other breeds (McKinley & Sambrook, 2000; Scott & Fuller, 1965), likely explaining the decreased no-choice trials by cooperative working breeds in the present study compared with noncooperative working breeds
It should be noted that handling of no-choice trials has been a challenge in OCT studies. A failure to respond within the allotted time may be due to distractions or loss of motivation, and not a failure to understand the cue. To prevent such instances from resulting in misleading point-following accuracy, no-choice trials are sometimes repeated or omitted. However, repeating trials can lead to within-session learning, and omitting trials can complicate analyses and interpretation of the data (Udell, Dorey, & Wynne, 2010b). Alternatively, as in the present study, no-choice trials are sometimes coded as incorrect with the assumption that anything other than choosing the correct container reflects a failure to comprehend and respond to the cue (Lazarowski & Dorman, 2015; Udell & Wynne, 2010). In this case, motivation trials should be included to eliminate potential confounds of loss of motivation causing erroneous incorrect responses. Our further categorization of incorrect responses as having actually chosen the incorrect box or failed to make a response allowed for further analyses of the occurrence of no choice trials. The fact that we found effects of group and breed on no-choice trials suggests that these types of responses may still be valuable and should be considered when evaluating performance.
Differences between pet and detection dogs also emerged in the UT. Pet dogs spent more time engaged in human-oriented gazing than did detection dogs, and showed preferential gazing toward the familiar human. This finding contrasts with previous reports that dogs trained for working roles or other tasks showed increased human-directed gazing in the unsolvable task compared with pets (D’Aniello et al., 2015; Marshall-Pescini et al., 2009). However, dogs in these studies were trained for roles involving a high degree of contact and cooperation with a human handler, such as SAR and agility, whereas detection training fosters independence with little dog–human coordination. In this view, our results are in line with reports that guide dogs, also trained for a relatively high level of independence, showed decreased levels of human-directed gazing (Scandurra et al., 2015). However, we recently found that while human-directed gazing in the UT in a cohort of young candidate detection dogs in training was low overall, dogs that did exhibit the behavior were more likely to be selected for service as a detection dog in the future (Lazarowski, Strassberg, Waggoner, & Katz, 2019b). We interpreted this finding to suggest that human-directed gazing reflected dogs’ flexibility in problem-solving strategies (i.e., a lack of maladaptive perseveration) and a degree of sociability needed for trainability and cooperation with a handler. Taken together, training and experience appear to influence human-directed gazing during the unsolvable task, with differential effects depending on the nature of the training and corresponding degree of human interaction involved. Although past studies suggest that level of attachment does not differ substantially between pet dogs and privately owned dogs trained for working roles (Mariti et al., 2013; Scandurra, Alterisio, & D’Aniello, 2016), future research could examine whether individual level of attachment to the owner, particularly in working dogs with less human-involved work, mediates differences in human-oriented behavior in the UT.
Unlike the OCT, we did not find that human-directed gazing was greater in cooperative working breeds in the UT. This result contrasts previous studies showing that dogs from hunting and herding breeds (e.g., border collies and golden retrievers), selectively bred for working in concert with people, gazed at humans during the UT more often than did dogs from breeds that did not experience selection for such traits (Konno et al., 2016; Passalacqua et al., 2011). However, consistent with past studies (Lazarowski, Strassberg, et al., 2019b; Passalacqua et al., 2011; Persson, Roth, Johnsson, Wright, & Jensen, 2015), we did find that human-directed gazing increased with age, supporting the hypothesis that human-directed communicative behavior is influenced by cumulative experience with humans.
We also found differences between pet and detection dogs in persistence during the UT, where detection dogs spent a greater amount of time in physical contact with the apparatus than did pet dogs. This result is consistent with findings of increased persistence in problem-solving tasks as a function of training history, again attributed to effects of promoting independent problem solving (Marshall-Pescini, Frazzi, & Valsecchi, 2016; Marshall-Pescini et al., 2008; Scandurra et al., 2015). Thus, differences in human-directed gazing between pet and detection dogs may have actually been driven by differences in persistence, where gazing may have been an artifact of giving up sooner. Indeed, recent studies have suggested that the UT measures persistence (or lack thereof) rather than “help seeking” by comparing looking behavior during baseline measures and trials in which other nonhuman stimuli were present (Lazzaroni et al., 2020); however, in the current study, we did not include a baseline phase. Decreased persistence by pets in the UT could also be a result of them having a history of being punished for manipulating food containers (Brubaker & Udell, 2018).
Persistence is likely especially important for detection dogs, who often work for long periods of time and in extreme environments in which rate of reinforcement is low, and may be indicative of resistance to extinction (Hall, 2017). Given recent evidence of high levels of persistence in candidate detection dogs present from an early age (Lazarowski, Strassberg, et al., 2019b), persistence may reflect a behavioral trait that has been selected for in dogs required to perform complex tasks. However, recent studies examining UT performance in detection dogs did not find an association between individual differences in persistence and measures of detection-dog performance (Lazarowski, Strassberg, et al., 2019b; MacLean & Hare, 2018; Tiira, Tikkanen, & Vainio, 2020). Future research is needed to identify the mechanisms underlying persistence that may result in individual differences in levels of persistence (e.g., motivation and reward value) as well as methods to evaluate aspects of persistence that are important for working-dog success.
Finally, we found no correlation between OCT and UT performance. This finding is consistent with the only other study we are aware of that compared OCT and UT performance, also finding no relationships (Sundman et al., 2018). Based on these findings, responding to human communicative cues and emitting human-directed communicative cues in problem-solving contexts appear to reflect two separate mechanisms. Another possibility that has been recently raised by researchers is that the human-directed gazing behavior observed in the UT does not reflect social behavior (Sundman et al., 2018), but rather results from giving up and looking at the most salient stimulus in the environment (Lazzaroni et al., 2020). Thus, the increased human-directed gazing in pets compared with detection dogs in our study likely resulted from the detection dogs spending more time persisting, and pets’ preferential gazing toward their owner, compared with the unfamiliar experimenter, may reflect a history of associating their owner/gazing at their owner with food (Lazzaroni et al., 2020). There is also evidence that gazing at a person during a novel or potentially stressful situation reflects social referencing, attempting to gain information about how to approach the situation, and that previous training involving taking instruction from a handler may influence social referencing behavior (Merola, Marshall-Pescini, D’Aniello, & Prato-Previde, 2013). In this view, it makes sense that OCT and UT behaviors do not correlate.
There are some important limitations to the current study. First, breeds were not matched between groups, resulting in the detection-dog group consisting almost entirely of traditional working breeds, whereas the pet-dog group was mixed. However, including breed group as a factor allowed us to control for this variable. Thus, differences that emerged between groups but not between breed (e.g., human-directed gazing in the UT) or vice versa (e.g., point-following in the OCT) are attributable to the corresponding variable. That is, although we found that cooperative working breeds performed better than other breeds in the OCT, we did not find that the detection-dog group (which consisted almost entirely of cooperative working breeds) performed better than the pet group. Our pet sample did not include a sufficient number of breeds belonging to independent worker breeds, and thus all noncooperative worker breeds were grouped together for the purposes of our analyses. Had we included more and different types of breed groups, more intricate findings related to breed history may have emerged.
A related limitation is that we cannot conclude whether differences between the two samples in our study were due solely to training or to genetics. That is, procurement of detection dogs typically involves selection for particular behavioral characteristics suitable for detection tasks (Jamieson et al., 2017); thus, differences between groups may have been due to preexisting behavioral traits or training experienced after selection, or a combination of both. Regardless, differences between the groups suggests that pet and detection dogs differ in sociocognitive abilities, likely reflecting the nature of their role, regardless of causal mechanism.
Another limitation is that pet dogs in our study lived in human homes, whereas detection dogs resided in kennels at their respective training centers. This could potentially explain group differences in human-directed gazing in the UT, where the familiarity of the owner, with whom the pet dogs resided in the same home, is greater than the familiarity of the handler. However, the interaction between detection dogs and handler that occurs during training could arguably be more significant than pets whose owners spend a significant portion of their day at work and do not engage in much interaction. Future studies could attempt to further quantify level of familiarity, for example by measuring average amount and quality of daily interaction between the dog and the person.
Thus, it could be argued that differences between groups were due to environmental conditions and not group-specific experience, per se. However, detection dogs performed just as well as pets in the OCT suggesting that residing in a kennel environment did not adversely affect their problem-solving abilities or ability to engage in a social task with humans. This finding is likely due to the intense training and frequent interactions between the detection dogs and their trainers, as opposed to other populations of kenneled dogs with limited human interaction who have shown to perform poorly on the OCT (D’Aniello et al., 2017; Lazarowski & Dorman, 2015; Udell et al., 2010a). Previous reports of decreased human-directed gazing in the UT by kenneled dogs compared with pet dogs point to possible effects of increased human interaction on the development of gazing behavior (D’Aniello & Scandurra, 2016); however, other findings again suggest that this difference is due to selection and/or training for more independent problem solving (i.e., persistence) driving a decrease in human-directed gazing (Scandurra et al., 2015). While the discrepancy in living conditions in our study was virtually unavoidable given that working dogs enrolled in training programs commonly reside in kennels, future studies could test deployed detection dogs residing with their handler, as in Scandurra et al. (2015), who compared guide dogs in training versus those paired in a home. Testing detection dogs that have been paired with a handler may also reveal effects of familiarity of the person not observed here. Additionally, future research should examine effects of rearing or current environment on working dogs’ sociocognitive abilities by comparing various alternative rearing strategies (e.g., prison puppy programs and volunteer puppy-raising foster homes).
Other limitations inherent to working with community-sourced dogs are unknown factors regarding the dogs’ life history, such as the number of previous owners and early life experiences. Our stipulation for including dogs in the pet group was that they had been living in the home of their current owner for a minimum of 1 year, but we did not attempt to quantify pet dogs’ training experience (e.g., obedience or sport) as a factor. While it is likely that the pet-dog group represented a mix of pets with and without formal training, this factor would be difficult to control given that many pets are adopted from shelters or rescue groups, and therefore their history prior to adoption would be unknown. To control for this factor, future studies including pets should exclude those for which the owner does not have a complete record of the dogs’ prior history (e.g., pets sourced from a breeder or raised by the owner).
In conclusion, our results suggest that differences in social cognition may exist as a function of dogs’ occupational relationship with humans and the specific nature of the role. Whether these differences are due to selection or experience remains to be explored. We also found differences in performance of the two groups between the two tasks, suggesting that the OCT and UT measure different aspects of social cognition, and that these differences between groups is task dependent.
References
Bates, D., Mächler, M., Bolker, B. M., & Walker, S. C. (2015). Fitting linear mixed-effects models using lme4. Journal of Statistical Software, 67(1). doi:https://doi.org/10.18637/jss.v067.i01
Brubaker, L., & Udell, M. A. R. (2018). The effects of past training, experience, and human behaviour on a dog’s persistence at an independent task. Applied Animal Behavior Science doi:https://doi.org/10.1016/j.applanim.2018.04.003
Call, J., Bräuer, J., Kaminski, J., & Tomasello, M. (2003). Domestic dogs (Canis familiaris) are sensitive to the attentional state of humans. Journal of Comparative Psychology (Washington, DC: 1983), 117(3), 257–263. doi:https://doi.org/10.1037/0735-7036.117.3.257
Cavalli, C. M., Carballo, F., Dzik, M. V., & Bentosela, M. (2019). Gazing as a help requesting behavior: A comparison of dogs participating in animal-assisted interventions and pet dogs. Animal Cognition. doi:https://doi.org/10.1016/j.jveb.2019.07.008
Cunningham, C. L., & Ramos, M. F. (2014). Effect of training and familiarity on responsiveness to human cues in domestic dogs (Canis familiaris). Animal Cognition, 17(3), 805–814. doi:https://doi.org/10.1007/s10071-013-0714-z
D’Aniello, B., Scandurra, A., Prato-Previde, E., & Valsecchi, P. (2015). Gazing toward humans: A study on water rescue dogs using the impossible task paradigm. Behavioural Processes, 110, 68–73. doi:https://doi.org/10.1016/j.beproc.2014.09.022
D’Aniello, B., Alterisio, A., Scandurra, A., Petremolo, E., Iommelli, M. R., & Aria, M. (2017). What’s the point? Golden and Labrador retrievers living in kennels do not understand human pointing gestures. Animal Cognition, 20(4), 777–787. doi:https://doi.org/10.1007/s10071-017-1098-2
D’Aniello, B. D., & Scandurra, A. (2016). Ontogenetic effects on gazing behaviour: A case study of kennel dogs (Labrador Retrievers) in the impossible task paradigm. Animal Cognition doi:https://doi.org/10.1007/s10071-016-0958-5
Gácsi, M., McGreevy, P., Kara, E., & Miklósi, A. (2009). Effects of selection for cooperation and attention in dogs. Behavioral and Brain Functions, 5, 31. doi:https://doi.org/10.1186/1744-9081-5-31
Hall, N. J. (2017). Persistence and resistance to extinction in the domestic dog: Basic research and applications to canine training. Behavioural Processes, 141, 67–74. doi:https://doi.org/10.1016/j.beproc.2017.04.001
Heberlein, M.T.E., Turner, D.C., Range, F., Virányi, Z. (2016) A comparison between wolves, Canis lupus, and dogs, Canis familiaris, in showing behaviour towards humans. Animal Behaviour, 122, 59–66.
Hare, B., Call, J., & Tomasello, M. (1998). Communication of food location between human and dog (Canis Familiaris). Evolution of Communication, 21, 137–159. doi:https://doi.org/10.1075/eoc.2.1.06har
Hare, B., Brown, M., Williamson, C., & Tomasello, M. (2002). The domestication of social cognition in dogs. Science (New York, N.Y.), 298(5598), 1634–1636. doi:https://doi.org/10.1126/science.1072702
Helton, W. S. (2009). Canine ergonomics: The science of working dogs. Boca Raton, FL: CRC Press.
Huber, L., Popovová, N., Riener, S., Salobir, K., & Cimarelli, G. (2018). Would dogs copy irrelevant actions from their human caregiver? Learning & Behavior, 1–11. doi:https://doi.org/10.3758/s13420-018-0336-z
Jamieson, L. T. J., Baxter, G. S., & Murray, P. J. (2017). Identifying suitable detection dogs. Applied Animal Behaviour Science doi:https://doi.org/10.1016/j.applanim.2017.06.010
Jarvis, T., & Hall, N. J. (2020). Development of point following behaviors in shelter dogs. Learning & Behavior doi:https://doi.org/10.3758/s13420-020-00415-8
Kaminski, J., & Nitzschner, M. (2013). Do dogs get the point? A review of dog-human communication ability. Learning and Motivation, 44(4), 294–302. doi:https://doi.org/10.1016/j.lmot.2013.05.001
Lakatos, G., Soproni, K., Doka, A., & Miklósi, A. (2009). A comparative approach to dogs’ (Canis familiaris) and human infants’ comprehension of various forms of pointing gestures. Animal Cognition, 12, 621–631.
Lazarowski, L., & Dorman, D. C. (2015). A comparison of pet and purpose-bred research dog (Canis familiaris) performance on human-guided object-choice tasks. Behavioural Processes, 110, 60–67. https://doi.org/10.1016/j.beproc.2014.09.021
Lazarowski, L., Haney, P., Brock, J., Fischer, T., Rogers, B., Angle, C., … Waggoner, L. P. (2018). Investigation of the behavioral characteristics of dogs purpose-bred and prepared to perform Vapor Wake® detection of person-borne explosives. Frontiers in Veterinary Science, 5, 50. https://doi.org/10.3389/fvets.2018.00050
Lazarowski, L., Rogers, B., Waggoner, L. P., & Katz, J. S. (2019a). When the nose knows: Ontogenetic changes in detection dogs’ (Canis familiaris) responsiveness to social and olfactory cues. Animal Behaviour, 153, 61–68.
Lazarowski, L., Strassberg, L. R., Waggoner, L. P., & Katz, J. S. (2019b). Persistence and human-directed behavior in detection dogs: Ontogenetic development and relationships to working dog success. Applied Animal Behaviour Science, 220. doi:https://doi.org/10.1016/j.applanim.2019.104860
Lazzaroni, M., Marshall, S., Helena, P., Sarah, M., Lucy, G., Darc, L., … Range, F. (2020). Why do dogs look back at the human in an impossible task? Looking back behaviour may be over-interpreted. Animal Cognition. doi:https://doi.org/10.1007/s10071-020-01345-8
Lit, L., Schweitzer, J. B., & Oberbauer, A. M. (2011). Handler beliefs affect scent detection dog outcomes. Animal Cognition, 14(3), 387–394. doi:https://doi.org/10.1007/s10071-010-0373-2
MacLean, E., & Hare, B. (2018). Enhanced selection of assistance and explosive detection dogs using cognitive measures. Frontiers in Veterinary Science, 5(October), 236. doi:https://doi.org/10.3389/FVETS.2018.00236
Mariti, C., Ricci, E., Carlone, B., Moore, J. L., Sighieri, C., & Gazzano, A. (2013). Dog attachment to man: A comparison between pet and working dogs. Journal of Veterinary Behavior: Clinical Applications and Research, 8(3), 135–145. doi:https://doi.org/10.1016/j.jveb.2012.05.006
Marshall-Pescini, S., Valsecchi, P., Petak, I., Accorsi, P. A., & Previde, E. P. (2008). Does training make you smarter? The effects of training on dogs’ performance (Canis familiaris) in a problem solving task. Behavioural Processes, 78(3), 449–454. doi:https://doi.org/10.1016/j.beproc.2008.02.022
Marshall-Pescini, S., Passalacqua, C., Barnard, S., Valsecchi, P., & Prato-Previde, E. (2009). Agility and search and rescue training differently affects pet dogs’ behaviour in sociocognitive tasks. Behavioural Processes, 81(3), 416–422. doi:https://doi.org/10.1016/j.beproc.2009.03.015
Marshall-Pescini, S., Frazzi, C., & Valsecchi, P. (2016). The effect of training and breed group on problem-solving behaviours in dogs. Animal Cognition, 19(3), 571–579. doi:https://doi.org/10.1007/s10071-016-0960-y
McKinley, J., & Sambrook, T. D. (2000). Use of human-given cues by domestic dogs (Canis familiaris) and horses (Equus caballus). Animal Cognition, 3(1), 13–22. doi:https://doi.org/10.1007/s100710050046
Merola, I., Marshall-Pescini, S., D’Aniello, B., & Prato-Previde, E. (2013). Social referencing: Water rescue trained dogs are less affected than pet dogs by the stranger’s message. Applied Animal Behaviour Science, 147, 132–138.
Miklósi, A. (2015). Dog behaviour, evolution, and cognition (2nd). Oxford, England: Oxford University Press.
Miklósi, Á., Polgárdi, R., Topál, J., & Csányi, V. (1998). Use of experimenter-given cues in dogs. Animal Cognition, 1, 113–121.
Mongillo, P., Bono, G., Regolin, L., & Marinelli, L. (2010). Selective attention to humans in companion dogs, Canis familiaris. Animal Behaviour, 80(6), 1057–1063. doi:https://doi.org/10.1016/j.anbehav.2010.09.014
Müller, C. A., Schmitt, K., Barber, A. L. A., & Huber, L. (2015). Dogs can discriminate emotional expressions of human faces. Current Biology, 25(5), 601–605. doi:https://doi.org/10.1016/j.cub.2014.12.055
Passalacqua, C., Marshall-Pescini, S., Barnard, S., Lakatos, G., Valsecchi, P., & Prato Previde, E. (2011). Human-directed gazing behaviour in puppies and adult dogs, Canis lupus familiaris. Animal Behaviour, 82(5), 1043–1050. doi:https://doi.org/10.1016/j.anbehav.2011.07.039
Persson, M. E., Roth, L. S. V, Johnsson, M., Wright, D., & Jensen, P. (2015). Human-directed social behaviour in dogs shows significant heritability. Genes, Brain and Behavior, 14(4), 337–344. doi:https://doi.org/10.1111/gbb.12194
Pongrácz, P., Miklósi, Á., Kubinyi, E., Gurobi, K., Topál, J., & Csányi, V. (2001). Social learning in dogs: The effect of a human demonstrator on the performance of dogs in a detour task. Animal Behaviour, 62(6), 1109–1117. doi:https://doi.org/10.1006/anbe.2001.1866
Reid, P. J. (2009). Adapting to the human world: Dogs’ responsiveness to our social cues. Behavioural Processes, 80(3), 325–333. doi:https://doi.org/10.1016/j.beproc.2008.11.002
Rooney, N. J., Bradshaw, J. W. S., & Almey, H. (2004). Attributes of specialist search dogs—A questionnaire survey of UK dog handlers and trainers. Journal of Forensic Sciences, 49(2), 300–306. doi:https://doi.org/10.1520/JFS2003048
Scandurra, A., Prato-Previde, E., Valsecchi, P., Aria, M., & D’Aniello, B. (2015). Guide dogs as a model for investigating the effect of life experience and training on gazing behaviour. Animal Cognition, 937–944. doi:https://doi.org/10.1007/s10071-015-0864-2
Scandurra, A., Alterisio, A., & D’Aniello, B. (2016). Behavioural effects of training on water rescue dogs in the Strange Situation Test. Applied Animal Behaviour Science, 174, 121–127. doi:https://doi.org/10.1016/j.applanim.2015.10.007
Scandurra, A., Alterisio, A., Marinelli, L., Mongillo, P., Semin, G. R., & D’Aniello, B. (2017). Effectiveness of verbal and gestural signals and familiarity with signal-senders on the performance of working dogs. Applied Animal Behaviour Science, 191, 78–83. doi:https://doi.org/10.1016/j.applanim.2017.02.003
Scott, J. P., & Fuller, J. L. (1965). Genetics and the social behavior of the dog. Chicago, IL: University of Chicago Press.
Soproni, K., Miklósi, A., Topál, J., & Csányi, V. (2001). Comprehension of human communicative signs in pet dogs (Canis familiaris). Journal of Comparative Psychology, 115(2), 122–126. doi:https://doi.org/10.1037/0735-7036.115.2.122
Sundman, A.-S., Persson, M. E., Grozelier, A., Halldén, L.-L., Jensen, P., & Roth, L. S. V. (2018). Understanding of human referential gestures is not correlated to human- directed social behaviour in Labrador retrievers and German shepherd dogs. Applied Animal Behaviour Science, 201, 46–53. doi:https://doi.org/10.1016/j.applanim.2017.12.017
Tiira, K., Tikkanen, A., & Vainio, O. (2020). Inhibitory control—Important trait for explosive detection performance in police dogs? Applied Animal Behaviour Science, 104942. doi:https://doi.org/10.1016/j.applanim.2020.104942
Udell, M. A. R., & Wynne, C. D. L. (2010). Ontogeny and phylogeny: both are essential to human-sensitive behaviour in the genus Canis. Animal Behaviour, 79(2), e9–e14. doi:https://doi.org/10.1016/j.anbehav.2009.11.033
Udell, M. A. R., Dorey, N. R., & Wynne, C. D. L. (2008a). Wolves outperform dogs in following human social cues. Animal Behaviour, 76(6), 1767–1773. doi:https://doi.org/10.1016/j.anbehav.2008.07.028
Udell, M. A R., Giglio, R. F., & Wynne, C. D. L. (2008b). Domestic dogs (Canis familiaris) use human gestures but not nonhuman tokens to find hidden food. Journal of Comparative Psychology (Washington, DC: 1983), 122(1), 84–93. doi:https://doi.org/10.1037/0735-7036.122.1.84
Udell, M. A. R., Dorey, N. R., & Wynne, C. D. L. (2010a). The performance of stray dogs (Canis familiaris) living in a shelter on human-guided object-choice tasks. Animal Behaviour, 79(3), 717–725. doi:https://doi.org/10.1016/j.anbehav.2009.12.027
Udell, M. A. R., Dorey, N. R., & Wynne, C. D. L. (2010b). What did domestication do to dogs? A new account of dogs’ sensitivity to human actions. Biological Reviews of the Cambridge Philosophical Society, 85(2), 327–345. doi:https://doi.org/10.1111/j.1469-185X.2009.00104.x
Udell, M. A. R., Ewald, M., Dorey, N. R., & Wynne, C. D. L. (2014). Exploring breed differences in dogs (Canis familiaris): Does exaggeration or inhibition of predatory response predict performance on human-guided tasks? Animal Behaviour, 89, 99–105. doi:https://doi.org/10.1016/j.anbehav.2013.12.012
Werhahn, G., Virányi, Z., Barrera, G., Sommese, A., & Range, F. (2016). Wolves (Canis lupus) and dogs (Canis familiaris) differ in following human gaze into distant space but respond similar to their packmates’ gaze. Journal of Comparative Psychology, 130(3), 288–298. doi:https://doi.org/10.1037/com0000036
Wobber, V., Hare, B., Koler-Matznick, J., Wrangham, R., & Tomasello, M. (2009). Breed differences in domestic dogs’ (Canis familiaris) comprehension of human communicative signals. Interaction Studies, 10(2), 206–224. doi:https://doi.org/10.1075/is.10.2.06wob
Zubedat, S., Aga-Mizrachi, S., Cymerblit-Sabba, A., Shwartz, J., Leon, J. F., Rozen, S., … Avital, A. (2014). Human–animal interface: The effects of handler’s stress on the performance of canines in an explosive detection task. Applied Animal Behaviour Science, 158, 69–75. doi:https://doi.org/10.1016/j.applanim.2014.05.004
Acknowledgements
This study was supported by grants from the APDT (Association of Professional Dog Trainers) Foundation to L.L. (2017 Dr. R.K. Anderson Grant Cycle), the Auburn University Graduate School to A.T., and the DARPA (Defense Advanced Research Projects Agency) government contract/grant number W911QX-13-C-0123 to G.D. The views, opinions, and/or findings contained in this article are those of the authors and should not be interpreted as representing the official views or policies, either expressed or implied, of the Defense Advanced Research Projects Agency, U.S. Department of Defense or the federal Government of the United States.
We thank the trainers of ik9, LLC and Auburn University’s Canine Performance Sciences as well as the pet dog owners for participating in this study. We also thank members of the Comparative Cognition Lab for assistance with data collection and scoring, and the Auburn University MRI Research Center for accommodating behavioral testing. Portions of this study were part of A.T.’s doctoral dissertation.
Open practices statement
The data for all experiments are available as supplementary materials. Experiments were not preregistered.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lazarowski, L., Thompkins, A., Krichbaum, S. et al. Comparing pet and detection dogs (Canis familiaris) on two aspects of social cognition. Learn Behav 48, 432–443 (2020). https://doi.org/10.3758/s13420-020-00431-8
Published:
Issue Date:
DOI: https://doi.org/10.3758/s13420-020-00431-8