Abstract
Sediments are carrier and a sink of elements in the hydrological cycle. Monitoring of contaminated soil and sediment with metallic elements is of interest due to their influence on groundwater, surface water, plants, animals and humans. In this study, different sediment samples from five various places were taken, and the elemental concentration along with some physiochemical parameters were determined. The elemental concentrations were determined with proton-induced X-ray emission, while the physiochemical parameters were determined using a conductivity meter, pH meter and thermometer. This study showed that silicon had highest mean concentration and lead had the lowest mean concentration. Cadmium had the highest contamination values in all the locations. Okitipupa had the highest pollution load index, and the lowest pollution index was recorded in Irele. The overall risk index (RI) in all the locations in the sediment indicated very high ecological risk index. Pollution load index (PLI) also categorized ecological risk which ranged from 8.812 to 28.42. Mean PLI value (17.7) recorded in this study was far higher than the threshold (< 1), indicating the presence of heavy pollutant levels. Such high-level PLI values signified danger and measures are needed in order to reduce the sources of pollutants in the sediment. From enrichment value and Igeo-accumulation values, Sn was severely polluted. Si, Zr, Ag and Cd were extremely polluted. The physiochemical parameters correlated with the elemental concentrations both negatively and positively.
Similar content being viewed by others
Introduction
Sediments had been explained by many authors as the fountain of numerous chemicals and also a source of water pollution (Celo et al. 1999; Mil-Homens et al.2013; Yu et al. 2001; Sakan et al. 2015; Wepener and Vermeulen (2005); Mil-Homens et al. 2007; Shakeri and Moor (2010); Yang et al. 2009). Sediment is the loose sand, clay, silt and other soil particles that settle at the bottom of body of water (Davies and Abowei 2009). Transportation of elements from sediments had been studied by some researchers in the laboratory scale and under environmental conditions (Sun et al. 2016; Vignati et al. 2013; Milacic et al. 2017). The quantification of trace elements in the sediments can be used to evaluate the pollution level and its relation with previous human activities (Thevenon and Pote 2012). The increase in industrialization and population has birthed dangerous chemicals and elemental pollutants that are always mobilized into the soils and sediments through numerous anthropogenic activities which include: industrialization, agricultural activities, atmospheric deposition and anthropogenic activities, where harmful chemicals and metals are generated. Soil is a reservoir of heavy metal which are repeatedly leached into the environment and sediments (Peizhong et al. 2015; Uwumarongie et al. 2008; Banat et al. 2005; Rafael et al. 2016); materials beneath surface water serve as a barometer to survey the pollutant level in aquatic ecosystem (Yang et al. 2009; Safaa 2015; Pekey et al. 2004). The physicochemical properties of sediments have pivotal role to play during the distribution of elements into water body (Covelo et al.2007; Hamidpour et al. 2010). Significant correlations were reported between metal contents with fine-grained particles of sediments, the total organic carbon (TOC) and pH (Zhang et al. 2014). The relative role of sediments’ physicochemical properties, metals’ availability and the possible ecological risk caused by the metal release had been discussed (Zhao et al. 2013). Recently, in Nigeria, the investigation of wide range of elements in the sediment using more sophisticated instrumentation such as X-ray fluorescence and inductively coupled plasma mass spectrometry had been undertaken (Ediagbonya and Ayedun (2018); Ayedun et al. 2019). This study seeks to ascertain the level of metallic elements using PIXE and some selected physiochemical parameters in sediments of five different rivers and to compare the value obtained in this study to the regulatory limits and other studies. There are various mechanisms by which sediments take up heavy metals. These may include biological uptake. However, it had been widely reported that heavy metals constitute nuisance in the sediment (Mateu et al. 1996; White et al. 2005; Birch et al. 2001).
Materials and methods
Study area
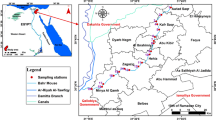
The samples were collected from towns and villages across three local government areas in the Southern Senatorial District of Ondo State Nigeria. The local government areas are: Ilaje Local Government, Irele Local Government and Okitipupa Local Government.
In Ilaje Local Government, samples were collected from Igbokoda. In Irele local government samples were collected from Ode-Irele, and from Okitipupa local government samples were collected from Igodan-Lisa. Farming remains the major occupation of the inhabitants of the three local government areas.
While the inhabitants of Ilaje are predominantly into fish farming being the local government with the longest coastline in Nigeria and an oil-producing area of the state, sizeable number of Ilaje is also into, mat weaving, boat building, etc. The inhabitants of Ode-Irele and Igodan-Lisa, in Okitipupa local government, are majorly cash crop and food crop farmers. The major cash crop predominantly planted by farmers is: palm trees that produce palm kernel and red oil that serve industrial and domestic consumption purposes. The common food crops in the areas of study are: yam, plantain, maize, cassava, etc. The three local government areas have deep rivers, but Ilaje local government is the only one among the three that has access to the sea.
The major source of pollution of rivers in these areas is through crude oil spillage caused by the effect of crude oil exploration in Ilaje local government area. Oil pollution has not only become a health challenge, it has also resulted to militancy and youth restiveness in the areas of study. Pollution of rivers and canals is mostly brought about by oil pipeline vandalization or accidents from oil companies operating in the areas. Oil bunkering is also a common feature in the areas. Aside from oil spillage, open defecation on rivers, swimming and sand evacuation from rivers for construction purposes also have a significant impact in the pollution of rivers in the areas of study. The map of the various locations is shown in Fig. 1 as reported by Ediagbonya and Gbolahan (2018).
Sample collection and preparation
A total of twenty-five (25) sediment samples were randomly collected in the study areas, and five (5) samples were taken at each sampling areas. The sediment samples were also air-dried for three days. The air-dried sediment samples were kept in polythene bag and then taking to the laboratory in Obafemi Awolowo University (Ife) for elemental and some physicochemical parameters analysis. Certified reference material (IAEA Soil-7) was used for as a quality control and quality assurance. The differences between the certified and measurement results were less than 15%.
Metal analysis
Proton-induced X-ray emission (PIXE), 1.7 MeV 5SDH Pelletron Accelerator, was used to analyze the elemental concentration. Detail of the analysis had reported by (Ediagbonya et al. 2020). The pH and electrical conductivity of the extracts were determined using portable pH and conductivity (Hanna 991300) meter. The nitrate and chloride were also determined as described by (Ademoroti 1996; Samira et al. 2009).
Geo-accumulation index (Igeo)
The geo-accumulation (Igeo) index is to evaluate contamination level in sediment as given by
(Müller 1969; Loska et al.1997). It had been used by (Ediagbonya and Ayedun (2018); AlKuziea 2015; Ayedun et al. 2019). Cn is the measured concentration of metal ‘n’ in sediments, and Bn is the background concentration of the same metal.
Enrichment factor (EF)
Enrichment factor (EF) was used to compute the extent of pollution in the sediment. To identify abnormal metal concentration in sediment in this study Al and Fe were used as reference elements. Few authors had used these elements reference element (Schiff and Weisberg (1999); Al-Khuziea et al. 2017. Al-Kuziea 2015). According to Ergin et al. (1991), the metal EF is defined as follows:
where X/Al is the ratio of the concentration heavy metal (X) to the Al concentration.
The reference crustal ratio of the shale value or the lithology was taken from Turekian (1961).
Pollution load index (PLI)
The pollution load index (PLI) is used to assess the level of elemental pollution in sediment. Detailed description had been reported by (Ayedun et al. 2019; Kwon and Lee 1998).
Ecological risk
The quantitative approach developed by Hakanson (1980) was used to calculate ecological risk factor as follows:
where Ti is the toxic response factor for a given substance (e.g., Cd = 30, Pb = 5, Cr = 2, Zn = 1 Cu = 5); Cf is the ecological risk factor; Ci represents metal content in the sediments; and Bi is the reference value.
Risk index (RI)
The overall risk index (RI) was calculated as the sum of potential ecological risk factor for heavy metals in sediments. Detailed report had been given by (Ayedun et al. 2019; Abdul-Kawi and Alhudify (2016); Mugosa et al. 2016).
Quality control
All reagents were of ultra-pure quality, and all laboratory wares used were thoroughly cleaned before utilization. All the samples were repeatedly measured (n = 5) to control the precision of analytical instruments.
Statistical data analysis
Statistical Package for Social Sciences (SPSS) version 24.0 was used for the statistical analyses in this work.
Results and discussion
Table 1 shows the descriptive statistics of physicochemical parameters in the different locations. The highest mean pH was recorded at Igodan 11.37 ± 0.38 (11.10-11.80), and the lowest recorded at Igbokoda 9.60 ± 0.10 (9.50-9.70). The highest mean (EC) was recorded at Igodan 4711.57 ± 0.31 (4711.30-4711.90) uS/cm, and lowest recorded at Erinje 2005.27 ± 0.21 (2005.10-2005.50) uS/cm. The highest mean temperature was recorded at Igbokoda 37.23 ± 0.35 (37.00-37.50), and lowest mean value reported at Erinje 31.53 ± 0.25 (31.30-31.80), highest mean chloride was recorded at Erinje 14.10 ± 0.00 (14.10-14.11) and lowest value reported at Igodan 9.6 ± 0.00 (9.66-9.66), while the highest nitrate was recorded at Igbokoda 2.71 ± 0.00 (2.71-2.71), and the lowest mean value reported at Erinje 0.97 ± 0.00 (0.97-0.97).
Table 2 shows the mean comparison of heavy metals in the different sampled location. The lowest mean Na level was recorded at Igbokoda (1415.4 ± 0.3), while the highest mean Na level was reported at Okitipupa. There was a significant spatial variation in the Na level in the different sampled locations. The different locations showed significantly spatial variations when compared with each other. The lowest mean Mg level was recorded at Igbokoda (2235.8 ± 0.2), while the highest mean Mg level was reported at Erinje (7952.3 ± 0.5). There was a significant spatial variation in the Mg level in the different sampled locations. The different locations showed significantly spatial variations when compared with each other. The lowest mean Al level was recorded at Okitipupa (2207.49 ± 1.5), while the highest mean Al level was reported at Erinje (290,934.5 ± 0.2). There was a significant spatial variation in the Al level in the different sampled locations. The different locations showed significantly spatial variations when compared with each other. The lowest mean Si level was recorded at Irele (501,037.4 ± 0.4), while the highest mean Si level was reported at Erinje (545,825.3 ± 0.4). There was a significant spatial variation in the Na level in the different sampled locations. The different locations showed significantly spatial variations when compared with each other. The lowest mean P level was recorded at Irele (269.6 ± 0.4), while the highest mean P level was reported at Erinje (7546.5 ± 0.3). There was a significant spatial variation in the different sampled locations. The different locations showed significantly spatial variations when compared with each other. The lowest mean Cl level was recorded at Igodan (188.5 ± 0.3), while the highest mean Cl level was reported at Irele (285.6 ± 0.4). There was a significant spatial variation in the different sampled locations. The different locations showed significantly spatial variations when compared with each other. The lowest mean K level was recorded at Igbokoda (2042.5 ± 0.4), while the highest mean K level was reported at Irele (8634.5 ± 0.4). There was a significant spatial variation in the different sampled locations. The different locations showed significantly spatial variations when compared with each other. The lowest mean Ca level was recorded at Okitipupa (2229.7 ± 0.3), while the highest mean Ca level was reported at Igbokoda (9018.6 ± 0.3). There was a significant spatial variation in the different sampled locations. The different locations showed significantly spatial variations when compared with each other. The lowest mean Ti level was recorded at Igodan (28,842.4 ± 0.4), while the highest mean Ti level was reported at Okitipupa (78,811.6 ± 0.3). There was a significant spatial variation in the different sampled locations. The different locations showed significantly spatial variations when compared with each other. The lowest mean Cr level was recorded at Irele (60.6 ± 0.3), while the highest mean Cr level was reported at Erinje (565.3 ± 3.2). There was a significant spatial variation in the different sampled locations. The different locations showed significantly spatial variations when compared with each other. The lowest mean Mn level was recorded at Irele (234.6 ± 0.2), while the highest mean Mn level was reported at Igbokoda (2064.4 ± 0.4). There was a significant spatial variation in the different sampled locations. The different locations showed significantly spatial variations when compared with each other. The lowest mean Fe level was recorded at Irele (20,088.4 ± 0.4), while the highest mean Fe level was reported at Erinje (63,973.3 ± 1.5). There was a significant spatial variation in the different sampled locations. The different locations showed significantly spatial variations when compared with each other. The lowest mean Cu level was recorded at Okitipupa (257.9 ± 0.4), while the highest mean Cu level was reported at Erinje (373.6 ± 0.3). There was a significant spatial variation in the different sampled locations. The different locations showed significantly spatial variations when compared with each other. The lowest mean Zn level was recorded at Irele (48.6 ± 0.3), while the highest mean Zn level was reported at Erinje (139.6 ± 0.2). There was a significant spatial variation in the different sampled locations. The different locations showed significantly spatial variations when compared with each other. The lowest mean Sr level was recorded at Okitipupa (46.5 ± 0.3), while the highest mean Sr level was reported at Erinje (317.5 ± 0.2). There was a significant spatial variation in the different sampled locations. The different locations showed significantly spatial variations when compared with each other. Rb was only reported at Irele with a mean value of 37.7 ± 0.6. The lowest mean Zr level was recorded at Irele (557.5 ± 0.2), while the highest mean Zr level was reported at Okitipupa (9156.7 ± 0.3). There was a significant spatial variation in the different sampled locations. The different locations showed significantly spatial variations when compared with each other. The lowest mean Ag level was recorded at Igbokoda (31.3 ± 1.5), while the highest mean Ag level was reported at Irele (73.6 ± 0.4). There was a significant spatial variation in the different sampled locations. The different locations showed significantly spatial variations when compared with each other. The lowest mean Cd level was recorded at Okitipupa (21.5 ± 0.4), while the highest mean Cd level was reported at Erinje (77.5 ± 0.3). There was a significant spatial variation in the different sampled locations. The different locations showed significantly spatial variations when compared with each other, but there was no significant difference in the mean Cd levels between Okitipupa and Igodan. The lowest mean Sn level was recorded at Okitipupa (71.2 ± 0.2), while the highest mean Sn level was reported at Irele (461.0 ± 1.0). There was a significant spatial variation in the different sampled locations. The different locations showed significantly spatial variations when compared with each other. The lowest mean Pb level was recorded at Igbokoda (10.3 ± 0.2), while the highest mean Pb level was reported at Erinje (12.5 ± 0.3). There was a significant spatial variation in the different sampled locations. The different locations showed significantly spatial variations when compared with each other, but there was no significant difference in the mean Pb levels between Okitipupa and Igbokoda. Bi was only recorded at Igodan (13.4 ± 0.1).
Sediments slowly heap up elements as well as other contaminants (Bing et al., 2013), which are often transported into the water bodies when there is change in environmental condition (Akcil et al., 2014). From Table 3, the concentration of sodium and magnesium in sediments from all locations ranged from 1415.4 to 7991.6 mg kg−1 and from 2235.8 to 7952.3 mg kg−1, respectively, with the mean values of 6294.4 and 4534.4 mg kg−1, respectively. The mean values of Na and Mg obtained from the present study were higher than the values that were reported in sediments of Lagos and Ogun State, Nigeria (Ayedun et al. 2019) but lower than the value reported by Alexakis 2008. The concentration of Na and Mg is higher in this study probably because of the larger amount of limestone and dolomite present in those sampling area. Aluminum is absorbed by living things in the water bodies and transported to human by food chain (Oberholster et al. 2012) and is badly taken by this living things from water bodies. In fish most especially aluminum clogs the gill of the fish when taken (Alstad et al. 2005). The availability of element in the water bodies is due to the pH of the water. Aluminum is also notorious for causing brain lesion which is known as Alzheimer’s disease (Lopez et al. 2002; Flaten 2001; Graves et al. 1990). The concentration of aluminum in sediments from all locations ranged from 220,749.2 to 290,934.5 ± 0.6 mg kg−1 with the mean value of 250,899.1 mg kg−1. The mean value of Al obtained from the present study was higher than the value that was reported in sediments of Lagos and Ogun State, Nigeria (Ayedun et al. 2019), and in Kolkata, India (Kumar et al. 2011). The higher value of Al in this study is probably due to the higher pH of water and organic matter content; these greatly influence the toxicity of aluminum. Researches had shown that Si is important in chicks and rats’ diet, and its deficit is associated with stunted growth, mostly with cartilage and bone formation, while in human being intake of silicon leads to exponential increase in bone mass (Refft et al. 1999; Birchall et al. 1996). The concentration of silicon in sediments from all locations ranged from 501,037.4 to 545,825.4 ± 0.8 with the mean values of 518,669.1 mg kg−1. Ejection phosphorus from bottom sediments is caused by numerous factors: pH, redox potential temperature, oxygen concentration, etc (Stephen et al. 1997; Søndergaard et al. 2017, 2002). Chlorine causes environmental harm at low levels. Chlorine is especially harmful to organisms living in water. The concentration of phosphorous and chlorine in sediments from all locations ranged from 269.6–7546.5 ± 0.8 to 188.5–285.6 ± 0.7, respectively, with the mean values of 3491.7 and 229.1 mg kg−1, respectively. The concentrations of these elements were not available in the other studies. The concentration of potassium, calcium and titanium in sediments from all locations ranged from 2042.5 to 8634.5 ± 0.8, 5190.4 to 35,661.4 ± 0.6 and 4561.4 to 78,811.6 ± 0.7 mg kg−1, respectively, with the mean values of 5318.8, 15,930.9 and 40,664.8 mg kg−1, respectively. The mean values of K, Ca and Ti obtained from the present study were higher than the values that were reported in sediments of Lagos and Ogun State, Nigeria (Ayedun et al. 2019) but lower than the values reported by Alexakis (2008). The concentration of K and Ca is higher in this study probably because the sediments in the sampling areas have more K and Ca occurring naturally in these areas. The concentration of chromium and manganese in sediments from all locations ranged from 60.6 to 565.3 ± 3.5, 234.6 to 2064.4 ± 0.6 mg kg−1, respectively, with the mean values of 388.2 and 1316.8 mg kg−1, respectively. The mean values of Mn obtained from the present study were lower than the values that were reported in sediments of Lagos and Ogun State, Nigeria (Ayedun et al. 2019), and higher than the values that were reported in sediments of Yemen, Korea and India (Abdul Kawi and Alhudify (2016); Kim et al. 2011; Suthar et al. 2009). Chromium is usually released by the electroplating, steel manufacturing, leather tanning and textile industries, causing surface water and well contamination which was subsequently found in sediments. Cr concentration is lower than that of Ayedun et al. (2019) because there are more steel manufacturing companies, textile industries, etc., in Lagos and Ogun state than in Ondo state, and the value is higher than that of Korea and India (Kim et al. 2011; Suthar et al.2009). The concentration of iron in the sediment from all the locations ranged from 20,088.4 to 63,973.3 ± 1.9. These concentrations of iron were relatively higher when compared to other studies both in Nigeria and in other countries (Ghrefat and Yusuf (2006); Cheggour et al. (2005); Ayedun et al. (2019); Kumar et al. 2011). The reason for this higher concentration could be due to high contamination of clay percentages, oil and gasoline spill to the water bodies in the studied area. Copper is an essential trace element for living organisms but can be harmful at high concentrations. Major causes of Cu contamination of water bodies include metal processing (Yi et al., 2011) and agriculture activities (El Azzi et al., 2013: Fontana et al., 2014).
The concentration of copper in sediments ranged from 257.9 to 373.6 ± 0.6 322.0. The mean value of Cu (322.0mgkg−1) obtained from the present study was higher than the values reported in other studies (Abdul Kawi and Alhudify (2016); Alexakis,2008; Rodríguez-Barroso et al. 2009). The higher concentration in this study could be as a result of environmental contamination (Kabta Pendias 2011). Lower concentration of zinc was recorded in this study when compared to other researches (Jadal et al. 2002;Moukrim et al. 2008;Mhamdi et al. 2010). The concentration of strontium and zirconium in sediments from all locations ranged from 46.5 to 317.5 ± 2.3, 557.5 to 9156.7 ± 0.5 mg kg−1, respectively, with mean values of 156.5, 37.7 and 5014.7 mg kg−1, respectively. The mean values of Sr and Zr obtained from this present study were lower than the values reported in sediments in Lagos and Ogun State, Nigeria (Ayedun et al.2019), and in East Attica, Greece (Alexakis,2008). The concentration of Zr was lower in this study probably due to the fact that there is no chemical industry around the sampling areas. The mean concentration of rubidium (37.7 ± 0.6 mg kg−1) which was also higher than the value reported by Alexakis (2008) but lower than the one reported by Butu and Iguisi (2013). The concentration of silver and bismuth in sediments from all locations ranged from 31.3 to 73.6 ± 1.9 and 13.4 ± 0.1, respectively, with the mean values of 45.6 and 13.4 mg kg−1, respectively. The concentrations of these heavy metals were not available in the other studies. Humans may get exposed to these metal primarily by inhalation and ingestion and can suffer from acute and chronic intoxication. Cadmium distributed in the environment will remain in soils and sediments for several decades. Agricultural activities had been implicated as the major man-made source of Cd contamination in aquatic environment (Illeperuma 2000; Jauasumana et al. 2011). The value of cadmium reported in this study was higher than other studies (Bullucci et al. 2003; Tahiri et al. 2005; Zheng et al. 2008). The concentration of tin and lead in sediments from all locations ranged from 71.2 to 461.0 ± 1.2, 10.3–12.5 ± 0.5 mg kg−1, respectively, with the mean values of 300.4 and 11.3 mg kg−1, respectively. The mean values of Sn and Pb obtained from the present study were higher than the values that were reported in sediments of Lagos and Ogun State, Nigeria (Ayedun et al. 2019) but lower than other studies in other regions (Amri et al. 2007;Kim et al. 2011). The high concentration of Pb in this study could be probably due to contamination from sewage discharges as well as oil enrichment.
Table 4 shows the correlation between physicochemical parameters and elements. Nitrate and chloride showed a negative significant relationship with Na, pH showed a positive significant relationship with Mg, and temperature and nitrate showed a negative significant relationship with Mg. pH is positively related to Al, pH, and chloride showed a positive relationship with Si. pH showed a positive relationship with P, EC, temperature, and nitrate showed a negative significant relationship with Cl. EC is significantly positively related to K, while nitrate is negatively related to K. pH showed a significant positive relationship with C. pH is positively related to Cr. pH and chloride are positively related to Fe. pH and chloride are positively related to Cu.
pH is positively related to Zn, and pH and chloride are positively related to Sr. pH has a significant negative perfect relationship with Rb, and EC has a significant negative relationship with Zr. EC shows a significant negative relationship with Ag, pH and chloride have a positive significant relationship with Cd, while temperature has a negative relationship with Cd. pH has a significant positive relationship with Sn, Pb, while temperature and nitrate have a negative significant relationship with Pb.
Figure 2 shows the component plot in rotated space. It gives a 3-dimensional plot of the principal components and the heavy metal loadings on the rotated space.
Table 5 shows the rotated component matrix. Each cell on the matrix gives the component loadings for each heavy metal. Mg, Al, P, Ca, Cr and Zn loaded highest under component 1, Ti, Mn, Zr, Ag, Sn and Pb loaded highest under component 2, Si, Fe, Cu, Sr and Cd loaded highest under component 3, while Na, Cl, K and Cu loaded highest under component 4. Metals that loaded highest under similar components are indicative of similar source.
Enrichment factor values were taken as suggested by Sutherland (2000) for the metals studied (Kabata and Pendias 1999). EF < 1 indicates no enrichment, EF < 3 is minor enrichment, EF = 3–5 is moderate enrichment, EF = 5–10 is moderate to severe enrichment, EF = 10–25 is severe enrichment, EF = 25–50 is very severe enrichment, and EF > 50 is extremely severe enrichment. In Irele, when Al was used as reference element, Na, Mg, K, Ca, Cl, P, Sr, Rb, Pb, Ti, Mn, Fe, Zn and Cr indicated no enrichment. Si, Cu and Zr were minor enriched. Cd and Sn were very severe enriched, while Ag was extremely enriched. When Fe was used as reference element, Mg, P, K, Ca, Mn, Sr, Rb, Zr indicated no enrichment. Na, Pb, Cr and Zn were minor enrichment. Cl was moderate enrichment. Al and Zr were moderate to severe enrichment. Ag, Cd and Sn were extremely enriched. When silicon was used as a reference element, Na, Mg, K, Ca, Cl, P, Sr, Rb, Pb, Ti, Mn, Fe, Al, Zn, Cu, Zn, Zr and Cr indicated no enrichment. Cd and Sn were moderate severe enrichment, while Ag was extremely enriched. In Okitipupa, when Al was used as reference element, Na, Mg, K, Ca, Cl, P, Sr, Pb, Mn, Fe and Zn indicated no enrichment. Si, Cr and Cu were minor enrichment. Ti and Sn were moderate to severe enrichment. Zr was severely enriched. Cd was severe enrichment, while Ag was extremely enriched. When Fe was Mg, K, Ca, Sr, Pb indicated no enrichment. Zn, P and Na were minor enrichment. Al and Mn were moderately enriched. Si, Cr and Cu were moderate severe enrichment. Ti and Sn were severe enrichment, while Zr, Ag and Cd were extremely severe enrichment. When Si was used in Okitipupa, Cd was severely enriched and Ag was extremely severe enrichment. In Erinje, when Al and Fe were used, Cd and Ag were extremely severe enrichment, but with Si, Ag only was extremely severe enrichment. In Igodan, when Al, Fe and Si were used as reference element, Ag, Cd and Sn were extremely severe enrichment. In Igbokoda, when Al, Fe and Si were used, Ag, Cd and Zr were extremely severe enrichment as shown in Table 6.
The original (Egrin et al. 1991) equation for the calculation of enrichment factor was substituted.
by using Al, Fe and Si as reference elements in this study. The order of sediments enrichment in Irele when Al was used as reference element was Ag > Cd > Sn > Si > Cu > Zr > Cl > Ti > Na > Cr > Pb > Zn > Fe > P > K > Mn > Rb > Mg > Ca > Sr.When Fe was used as the reference element, the order of sediment enrichment was Ag > Cd > Sn > Si > Cu > Zr > Al > Cl > Ti > Na > Cr > Pb > Zn > P > K > Mn > Rb > Mg > Ca > Sr. When Si was used as the reference element, the order of sediment enrichment was Ag > Cd > Sn > Cu > Zr > Al > Cl > Ti > Na > Cr > Pb > Zn > Fe > P > K > Mn > Rb > Mg > Ca > Sr, as shown in Table 6. The three reference elements (Al, Fe and Si) used in the computation of enrichment value showed similar trend as in decreasing order, but Fe showed the highest enrichment values when compared to Al and Si as reference element. These trends of enrichment value in this study can be compared to the trends obtained in other studies (Ali et al. 2015;Ayedun et al. 2019; Ediagbonya & Ayedun (2018). In Okitipupa, the trend showed a descending order of the enrichment value when Al was used as reference element; Ag > Cd > Zr > Ti > Sn > Si > Cu > Cr > Mn > P > Cl > Zn > Na > Fe > Pb > K > Mg > Sr > Ca, while Fe and Si showed the same pattern of descending enrichment value when it was used as reference elements. In Erinje, when Al was used as reference element, the enrichment value was in the order as: Ag > Cd > Sn > Zr > P > Cu > Ti > Si > Cr > Ca > Mn > Zn > Fe > Cl > Sr > Na > Pb > Mg, while Fe and Si also showed the similar pattern of decreasing enrichment value when it was used as reference elements. In Igodan, when Al was used as reference element, the enrichment value was in the order as: Ag > Sn > Cd > Zr > P > Cu > Si > Ti > Cr > Ca > Zn > Mn > Cl > Na > Sr > Pb > Mg > K, while Fe and Si also showed the same pattern of descending enrichment value when used as reference elements as shown in Table 6.
In Igbokoda, when Al was used as reference element, the enrichment value was in the order as: Ag > Cd > Zr > Sn > Ti > Cu > Si > Ti > Cr > P > Mn > Cl > Fe > Zn > Sr > Pb > Ca > Mg > Na > K. The same was observed when Fe and Si were used as reference element in Igbokoda.
Igeo ≤ 0 was classified as unpolluted, Igeo ≤ 1 was classified as slightly polluted, Igeo ≤ 2 was classified as moderately polluted, Igeo ≤ 3 was classified as moderately severely polluted, Igeo ≤ 4 was classified as severely polluted, Igeo ≤ 5 was classified as severely extremely polluted, and Igeo ≥ 5 was classified as extremely polluted. From Table 7, in Irele, the elements whose Igeo ≤ 0 were Na, Mg, P, K, Ca, Ti, Cr, Mn, Fe, Zn, Sr, Rb and Pb and they were classified as unpolluted. While Ag, Cd, Si and Sn classified as extremely polluted and their Igeo ≥ 5, Al and, Cl were classified as slightly polluted and their Igeo ≤ 1, the element Zr and Cu were moderately polluted. In Okitipupa, Na, Mg, Cl, K, Ca, Fe, Zn and Sr were slightly polluted, Cr was moderately polluted, Sn was moderately severely polluted, Ti was severely extremely polluted, while Si, Cd, Ag, Zr and Cu were extremely polluted. In Erinje, Na, Mg, Cl, Fe, Zn, Sr and Pb were unpolluted, and Ca and Mn were slightly polluted. Al was moderately polluted; P, Ti, Cr and Cu were moderately polluted. Zr was severely extremely polluted. Si, Ag, Cd and Sn were extremely polluted. In Igodan, Na, Mg, Cl, Ca, Mn, Fe, Zn, Sr were unpolluted. Al and Cr were moderately polluted. P, Ti, Cu and Zr were moderately severely polluted. Si, Ag, Cd and Sn were extremely polluted. In Igbokoda, Na, Mg, Cl, K, Ca, Fe, Zn, Sr and Pb were unpolluted. Al and Mn were slightly polluted. P and Cr were moderately polluted. Ti and Cu were moderately severely polluted. Sn was severely polluted. Si, Zr, Ag and Cd were extremely polluted. From Table 7, the contamination values for the few elements were: in Irele, Cd > Cu > Cr > Zn > Pb; in Okitipupa, Cd > Cu > Cr > Zn > Pb. In Erinje, Cd > Cu > Cr > Zn > Pb, in Igodan, Cd > Cu > Cr > Zn > Pb, in Igbokoda, Cd > Cu > Cr > Zn > Pb. The pollution load index was highest Okitipupa, and the lowest pollution index was recorded in Irele as shown in Table 7. The ecological risk values, in Irele, Cd > Pb > Cr > Zn; in Okitipupa, Cd > Cr > Pb > Zn. In Erinje, Cd > Cr > Pb > Zn, in Igodan, Cd > Cr > Pb > ZN, in Igbokoda, Cd > Cr > Pb > Zn as shown in Table 7. The ecological risk factor (Er i) for individual metals showed that Cd ranged from (6450 to 12,720) poses greater potential ecological risk followed by Cu (92–133.5), Cr (3.46–32.4), Pb (3.05–3.7) and Zn (0.92–2.63). Cadmium has no biological significance, and it is insidious to living organism as reported by (Alloway 1990). Toxic response factor (Tr i) for Cd was high (30) as compared to Tr i of Cr, Cu, Zn and Pb. The high toxic response factor (Tr i) for Cd was due to its relatively low background value (0.12) (Wei et al. 2015; CEPA and CGSEM 1990). Potential ecological risk factor for multimetal (RI) represents the sensitivity of various biological communities to toxic heavy metals. The overall risk index (RI) in all the locations in the sediment indicated very high ecological risk index. The values obtained in this study can be compared to the values obtained in other studies (Ali et al. 2015;Ayedun et al. 2019). Pollution load index (PLI) also categorized ecological risk, and the value obtained in this study in all the locations ranged from 8.812 to 28.42. Mean PLI value (17.7) recorded in this study was far higher than the threshold (< 1), indicating the presence of heavy pollutant levels. Such high-level PLI values signified danger and measures are needed in order to reduce the sources of pollutants in the sediment. The high pollution load, contamination value and ecological risk could be as result of soil erosion and indiscriminate dumping of solid waste, sewage and human into the river as well as oil spillage.
Figure 3 shows the distribution of physiochemical parameters in the water. Median, 25th and 75th percentiles are shown in the box; whiskers indicate the maximums and minimums.
Conclusion
In this study, different sediment samples from five different locations were analyzed for the presence of some elements along with some physiochemical parameters. The elemental concentration was determined using proton-induced X-ray emission (PIXE). The result showed that silicon and aluminum have the highest mean concentration with values of 518,669.1 and 250,899.1 mg kg−1, respectively, while lead and bismuth have the lowest mean concentration with values of 11.3 and 13.4 mg kg−1, respectively. Cadmium had the highest contamination values in all the locations. Okitipupa had the highest pollution load index, and the lowest pollution index was recorded in Irele. The overall risk index (RI) in all the locations in the sediment indicated very high ecological risk index. The mean PLI value (17.7) recorded in this study was far higher than the threshold (< 1), indicating the presence of heavy pollutant levels. Such high-level PLI values signified danger and measures are needed in order to reduce the sources of pollutants in the sediment. From enrichment value and Igeo-accumulation values, Sn was severely polluted. Si, Zr, Ag and Cd were extremely polluted
Change history
28 August 2020
In the print published article.
References
Abdul-Kawi AAA, Alhudify NS (2016) Assessment of heavy metals contamination and its ecological risk in the surface sediments of Al-Mukalla coast, Yemen. J Sci Eng Res 3(3):13–23
Ademoroti CMA (1996) Standard methods for water and effluent analysis. Ibadan Foludex press limited, pp 10–19
Akcil A, Erust C, Ozdemiroglu S, Fonti V, Beolchini F (2014) A review of approaches and techniques used in aquatic contaminated sediments: metal removal and stabilization by chemical and biotechnological processes. J Clean Prod 86:24–36
Alexakis D (2008) Geochemistry of stream sediments as a tool for assessing contamination by Arsenic, Chromium and other toxic elements: East Attica region, Greece. Eur Water 21(22):57–72
Ali Z, Malik RN, Shinwari ZK, Qadir A (2015) Enrichment, risk assessment, and statistical apportionment of heavy metals in tannery-affected areas. Int J Environ Sci Technol 12:537–550
Al-Khuzie DKK, Hassan WF, Al-Hatem Z, Abdulnabi ZA, AdilMizhi A, Shabar HA (2017) Use geo accumulation index and enrichment factor in assessing pollution in Iraqi tidal flats of some heavy metals. Indian J Nat Sci 7(40):11995–12005
Al-kuziea DKK (2015) Assessment of quality collected from shatt Al-Arab river, Basrah, southern Iraq. J Int Acad Res Multidicip 3(6):235–246
Alloway BJ (1990) Cadimium. In: Alloway BJ (ed) Heavymetals in soils. Blackie, Glagow, pp 100–124
Alstad NEW, Kjelsberg BM, Vøllestad A, Lydersen E, Polėo ABS (2005) The significance of water ionic strength on aluminium toxicity in brown trout (Salmo trutta L.). Environ Pollut 133(2):333–342
Amri N, Benslimane M, Zaoui H, Hamedoun M, Outiti B (2007) Evaluation of the heavy metals accumulate in samples of the sediments, soils and plants by Icp-oes with the average Sebou. M.J. Condensed Matter 8(1):43–52
Ayedun H, Arowolo TA, Gbadebo AM, Idowu OA (2019) Elemental enrichment of sediments in an unprotected shallow groundwater of Lagos and Ogun States, Nigeria. Environ Geochem Health 41(2):951–966
Banat KM, Howari FM, Al-Hamada AA (2005) Elements in urban soils of central Jordan: Should we worry about their environmental risks? Environ Res 97:258–273
Bing H, Wu Y, Nahm WH, Liu E (2013) Accumulation of heavy metals in the lacustrine sediment of Longgan Lake, middle reaches of Yangtze River, China. Environ Earth Sci 69:2679–2689
Birch GF, Taylor SE, Matthai C (2001) Small-scale spatial and temporal variance in the concentration of heavy metals in aquatic sediments: a review and some new concepts. Environ Pollut 113:357–372
Birchall J, Bellia J, Roberts N (1996) On the mechanisms underlying the essentiality of silicon–interactions with aluminium and copper. J Coord Chem 149:231–240
Butu AW, Iguisi EO (2013) Concentration of heavy metals in sediment of river Kubanni, Zaria, Nigeria. Compr J Environ Earth Sci 2:10–17
Celo V, Babi D, Baraj B, Cullaj A (1999) An assessment of heavy metal pollution in the sediments along the Albanian Coast. Water Air Soil Pollut 111:235–250
Cheggour C, Chafifik A, Fisher NS, Benbrahim S (2005) Metal concentrations in sediments and clams in four Moroccan estuaries. Mar Environ Res 59(2):119–137
Chinese Environmental Protection Agency and Chinese General Station of Environmental Monitoring (CEPA and CGSEM) (1990) Soil chemical element background values of China. Chinese Environmental Science Press, Beijing. (in Chinese)
Covelo EF, Vega FA, Andrade ML (2007) Heavy metal sorption and desorption capacity of soils containing endogenous contaminants. J Hazard Mater 143:419–430
Davies OA, Abowei JFN (2009) Sediment quality of lower reaches of Okpoka Creek, Niger Delta, Nigeria. Eur J Sci Res 26:437–442
Ediagbonya TF, Ayedun H (2018) Geochemistry of terrigenous sediments in surface water from ore and okitipupa southwest, Nigeria. Bangladesh J Sci Ind Res 53(2):145–154
Ediagbonya TF, Gbolahan OI (2018) Determination of radioactive isotopes and some physicochemical parameters in five different rivers in the south Ondo State, Nigeria. Int J Low Radiat 11(1):45–65
Ediagbonya TF, Ogunjobi JA, Olutayo OO (2020) Effect of quarry activities on selected biological resources around quarry site within Onigambari forest plantation, Oyo State, Nigeria. Environ Geochem Health 42(4):1–13
El Azzi D, Viers J, Guiresse M, Probst A, Aubert D, Caparros J, Charles F, Guizien K, Probst JL (2013) Origin and fate of copper in a small Mediterranean vineyard catchment: new insights from combined chemical extraction and δ65Cu isotopic composition. Sci Total Environ 463–464:91–101
Ergin M, Saydam CÖ, Baştürk Ö, Erdem E, Yörük R (1991) Heavy metal concentration in surface sediments from 2 inlets (Golden Horn Estuary and İzmit Bay) of the north eastern Sea of Marmara. Chem Geol 91:269–285
Flaten TP (2001) Aluminium as a risk factor in Alzheimer’s disease, with emphasis on drinking water. Brain Res Bull 55:187–196
Fontana LA, Albuquerque LS, Brenner M, Bonotto DM, Psabaris TP, Pires MAF, Cotrim MEB, Bicudo DC (2014) The eutrophication history of a tropical water supply reservoir in Brazil. J Paleolimnol 51:29–43
Ghrefat H, Yusuf N (2006) Assessing Mn, Fe, Cu, Zn and Cd pollution in bottom sediments of Wadi Al-Arab Dam, Jordan. Chemosphere 65:2114–2121
Graves AB, White E, Koepsell TD, Reifler BV, Belle G, Larson EB (1990) The association between aluminum–containing products and Alzheimer’s disease. J Clin Epidemiol 43:35–44
Hakanson L (1980) An ecological risk index for aquatic pollution control. A sedimentological approach. Water Res 14:975–1001
Hamidpour M, Kalbasi M, Afyuni M, Shariatmadari H, Holm PE, Hansen BCH (2010) Sorption hysteresis of Cd (II) and Pb(II) on natural zeolite and bentonite. J Hazard Mater 181:686–691
Illeperuma OA (2000) Environmental pollution in Sri Lanka: a review. J Natl Sci Found Sri Lanka 28:301–325
Jadal M, El Yachioui M, Bennasser L, Fekhaoui M, Foutlane A (2002) Water quality of the estuary of the Oued Oum Erbia (Morocco) and the influence of tidal dynamics. L’eau l’industrie les nuisances 256:59–66
Jauasumana MACS, Paranagama PA, Amarasinge M, Fonseka SJ, Wijekoon DV (2011) Presence of Arsenic in Pesticides Used in Sri Lanka. In: Water professional’s day symposium, Water Resources Research in Sri Lanka. Faculty of Agriculture, University of Peradenyia, pp 127–141
Kabata A, Pendias H (1999) Biochemistry of trace elements. Panstwowe Wydawnictwo Naukowe (in Polish), Warsaw
Kabata-Pendias A (2011) Trace elements in soils and plants, 4th edn. CRC Press, Boca Raton
Kim KT, Ra K, Kim ES, Yim UH, Kim JK (2011) Distribution of heavy metals in the surface sediments of the Han River and its estuary, Korea. J Coastal Res 64:903–907
Kumar B, Shah R, Mukherjee D (2011) Geochemical distribution of heavy metals in sediments from sewage fed fish ponds from Kolkata Wetlands. India Chem Speciat Bioavailab 23(1):24–32
Kwon YT, Lee CW (1998) Application of multiple ecological risk indices for the evaluation of heavy metal contamination in a coastal dredging area. Science Total Environ 214:203–210
López FF, Cabrera C, Lorenzo ML, López MC (2002) Aluminium content of drinking waters, fruit juices and soft drinks: contribution to dietary intake. Sci Total Environ 292:205–213
Loska K, Cebula J, Pelczar J, Wiechula D, Kwapulinski J (1997) Use of enrichment, and contamination factors together with geoaccumulation indexes to evaluate the content of Cd, Cu, and Ni in the Bybnik water reservoir in Poland. Water Air Soil Pollut 93:347–365
Mateu J, Forteza M, Colom-Altes M, Cerda V (1996) Atmospheric background levels and transport of heavy metals in the Balearic Islands. Water Air Soil Pollut 86:157–172
Mhamdi AA, Choura M, Maanan B, Zourarah B, Robin M (2010) Metal fluxes to the sediments of the Moulay Bousselham lagoon, Morocco. Environ Earth Sci 61:275–286
Milacic MR, Zuliani T, Vidmar J, Primozˇ Oprcˇkal P, Scancar J (2017) Potentially toxic elements in water and sediments of the Sava River under extreme flow events. Sci Total Environ 605–606:894–905
Mil-Homens M, Steven RL, Cato l, Abrantes F (2007) Regional geochemical baseline for Portuguese shelf sediments. Environ Pollut 148(2):418–427
Mil-Homens M, Costa AM, Fonseca S, Trancoso MA, Lopes C, Serrano R, Sousa R (2013) Natural heavy metal and metalloid concentration in sediments of the Minho River estuary (portugal): baseline values for environmental studies. Environ Monit Assess 185(7):5937–5950
Moukrim A, Chiffoleau JF, Burgeot T, Cheggou M (2008) Changes in the sediment trace metal contamination after the commissioning of a municipal wastewater treatment plant in the Souss Estuary (South Morocco). Bull Environ Contam Toxicol 80:549–554
Mugosa B, Durovi D, Mirjana Nedovi-Vukovi M, Labovi SB, Miroslav VM (2016) Assessment of ecological risk of heavy metal contamination in coastal municipalities of Montenegro. Int J Environ Res Public Health 13:393–398
Muller G (1969) Index of geoaccumulation in sediments of the Rhine River. Geol J 2:109–118
Oberholster PJ, Myburgh JG, Ashton PJ, Coetzee JJ, Botha AM (2012) Bioaccumulation of aluminium and iron in the food chain of Lake Loskop South Africa. Ecotoxicol Environ Saf 75:134–141
Peizhong L, Chunye L, Hongguang C, Xiaoli D, Kai L (2015) Contamination and health risk of soil heavy metal around a lead/zinc smelter in southwestern China. Ecotoxicol Environ Saf 113:391–399
Pekey H, Karakar D, Ayberk S, Tolun L, Bakogulu M (2004) Ecological risk assessment using trace elements from surface sediments of İzmir Bay (Northeastern Marmara Sea), Turkey. Mar Pollut Bull 48:946–953
Pszoniki L, Hanna AN, Suschny O (1984) Report on the intercomparison run IAEA-Soil-7:Trace elements in soil IAEA/RL/112 Vienna, Austria
Rafael A, Alejandro S, Fabian S, Helios P, Carmen F, Nuria G, Alejandro L, Enzo E (2016) Trace elements in centenarian, dodger. J Trace Elem Med Biol 35:103–106
Reffitt DM, Jugdaohsingh R, Thompson RP, Powell JJ (1999) Silicic acid: its gastrointestinal uptake and urinary excretion in man and effects on aluminium excretion. J Inorg Biochem 76:141–147
Rodríguez-Barroso RM, Benhamou Y, El-Moumni B, El Hatimi I, García-Morales JL (2009) Evaluation of metal contamination in sediments from north of Morocco: geochemical and statistical approaches. Environ Monit Assess 159(1–4):169–181
Safaa AAG (2015) Trace metals in seawater, sediments and some five fish species from Marsa Matrouh Beaches in north western Mediterranean coast, Egypt. Egypt J Aquat Res 41:145–154
Sakan S, Devic G, Relic D, And-elkovic I, Sakan N, Ðord-evic D (2015) Evaluation of sediment contamination with heavy metals: the importance of determining appropriate background content and suitable element for normalization. Environ Geochem Health 37:97–113
Samira ABM, Hawaa SE, Faiza AH, Fatma F (2009) Determination of available nitrate, phosphate and sulfate in soil samples. Int J Pharm Tech Res 1(3):598–604
Schiff KC, Weisberg SB (1999) Iron as a reference element for determining trace metal enrichment in southern california coastal shelf sediments. Mar Environ Res 48:161–176
Shakeri A, Moore F (2010) Impact of an industrial complex on freshly deposited sediments, Chener Rahdar river case study Shiraz, Iran. Environ Monit Assess 169(1–4):321–334
Søndergaard M, Lauridsen TL, Johansson L, Jeppesen E (2017) Nitrogen or phosphorus limitation in lakes and its impact on phytoplankton biomass and submerged macrophyte. Hydrobiologia 795:35–48
Stephen D, Moss B, Phillips G (1997) Do rooted macrophytes increase sediment phosphorus release? Hydrobiologia 342(343):27–34
Sun J, Quicksall AN, Chillrud SN, Mailloux BJ, Bostick BC (2016) Arsenic mobilization from sediments in microcosms under sulfate reduction. Chemosphere 153:254–261
Suthar S, Nema AK, Chabukdhara M, Gupta SK (2009) Assessment of metals in water and sediments of Hindon River, India: impact of industrial and urban discharges. J Hazard Mater 171(1–3):1088–1095
Sutherland RA (2000) Bed sediment-associated trace metals in an urban stream, Oahu, Hawaii. Environ Geol 39:611–627
Tahiri L, Bennasser L, Idrissi L, Fekhaoui M, El Abidi A (2005) Metal contamination of Mytilus galloprovincialis and sediment at the estuary Bouregreg (Morocco). Water Qual Res J Canada 40(1):111–119
Thevenon F, Pote J (2012) Water pollution history of Switzerland recorded by sediments of the large and deep perialpine lakes Lucerne and Geneva. Water Air Soil Pollut 223:6157–6169
Turekian KK, Wedepohl KH (1961) Distribution of elements in some majors units of the earth's crust. Geol Soc Am Bull 72:175–192
Uwumarongie EG, Igene HA, Ediagbonya TF (2008) Assessment of elements contaminated soil from automobile workshop in Benin City. Chem Tech J 4(1):90–95
Vepener V, Vermeulen LA (2005) A note on the concentrations and bioavailability of selected metals in sediments of Richards Bay Harbour, South Africa. Water SA 31(4):589–596
Vignati DAL, Secrieru D, Bogatova YI, Dominik J, Céréghino R, Berlinsky NA (2013) Trace element contamination in the arms of the Danube Delta (Romania/Ukraine): current state of knowledge and future needs. J Environ Manag 125:169–178
Wei G, Shouliang H, Beidou X, Jingtian Z, Fengchang W (2015) Heavy metal contamination in sediments from typical lakes in the fire geography regions of China: distribution, bioavailability and risk. Ecol Eng 81:243–255
White HK, Xu L, Lima ANL, EglitonT I, Reddy CM (2005) Abundance, composition and vertical transport of PAHs in marsh sediments. Environ Sci Technol 39:8273–8280
Yang Z, Wang Y, Shen Z, Niu J, Tang Z (2009) Distribution and speciation of elements in sediments from the mainstream, tributaries, and lakes of the Yangtze river catchment of Wuhan, China. J Hazard Mater 166(2–3):1186–1194
Yi Y, Yang Z, Zhang S (2011) Ecological risk assessment of heavy metals in sediment and human health risk assessment of heavy metals in fishes in the middle and lower reaches of the Yangtze River basin. Environ Pollut 159:2575–2585
Yu KC, Tsai LT, Chen SH, Pick FR (2001) Correlation analysis on ling behavior of elements with sediment matrices. Water Res 35(10):2417–2428
Zhang C, Yang MJ, Cui F, Zhu M, Hu LS (2014) Effect of sediment geochemical properties on heavy metal bioavailability. Environ Int 73:270–281
Zhao S, Feng C, Wang D, Liu Y, Shen Z (2013) Salinity increases the mobility of Cd, Cu, Mn, and Pb in the sediments of Yangtze Estuary: relative role of sediments’ properties and metal speciation. Chemosphere 91:977–984
Zheng N, Wang Q, Liang Z, Zheng D (2008) Characterization of heavy metal concentrations in the sediments of 3 freshwater rivers in Huludao City, Northeast China. Environ Pollut 154(1):135–142
Acknowledgements
We gratefully acknowledge the support of Heads of the Department Chemical Sciences for their logistic support. Also our sincere thanks goes to the people living in the various local government area for their immense assistance during the collection of the sediment samples and also the assistance rendered by Central Laboratories, located at the University of Ife, Nigeria, where the PIXE analysis was carried out.
Funding
Part of funding was from the institution, and it was Bsc Research Project.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Informed consent
It should be published.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ediagbonya, T.F., Balogun, O.T. Potential risk assessment and spatial distribution of elemental concentrations in sediment. Appl Water Sci 10, 176 (2020). https://doi.org/10.1007/s13201-020-01260-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13201-020-01260-w